All Photos(1)
About This Item
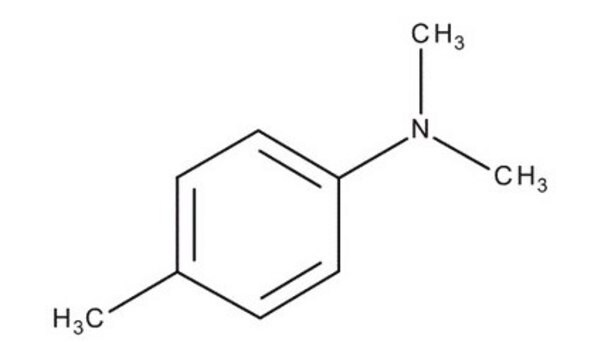
Linear Formula:
(CH3)3CC6H4N(CH3)2
CAS Number:
Molecular Weight:
177.29
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.529 (lit.)
bp
250-253 °C (lit.)
density
0.906 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CN(C)c1ccc(cc1)C(C)(C)C
InChI
1S/C12H19N/c1-12(2,3)10-6-8-11(9-7-10)13(4)5/h6-9H,1-5H3
InChI key
SJDILFZCXQHCRB-UHFFFAOYSA-N
General description
Potassium iodide catalyzed oxidative coupling of tert-butyl-N,N-dimethylaniline with indole has been investigated. It is an efficient polymerization accelerator.
Application
4-tert-Butyl-N,N-dimethylaniline was employed as amine initiator during free-radical/cationic hybrid photopolymerizations of acrylates and epoxides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Evaluation of initiator systems for controlled and sequentially curable free-radical/cationic hybrid photopolymerizations.
Oxman JD, et al.
Journal of Polymer Science: Part A, General Papers, 43(9), 1747-1756 (2005)
H Argentar et al.
Journal of the American Dental Association (1939), 102(5), 664-665 (1981-05-01)
These tests showed that DMBA, a recently commercialized amine accelerator, is more suitable from the standpoint of color for use in denture base, reline, and repair resins than is the commercially used amine, DMPT. As the curing times of all
Lan-Tao Li et al.
Organic & biomolecular chemistry, 10(48), 9519-9522 (2012-11-13)
A one-pot dual functionalization of indoles has been developed. The simultaneous C3-formylation and N-aminomethylation of indoles can be achieved using readily available potassium iodide as a catalyst and tert-butyl peroxybenzoate as a co-oxidant.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service