192171
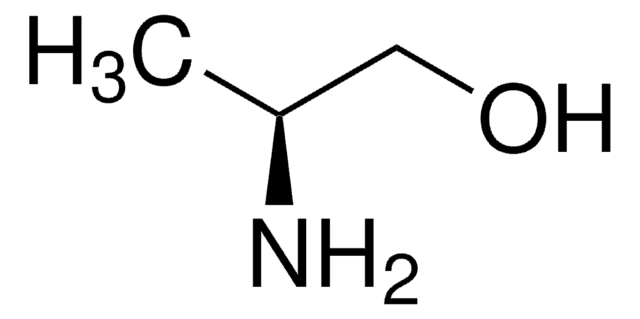
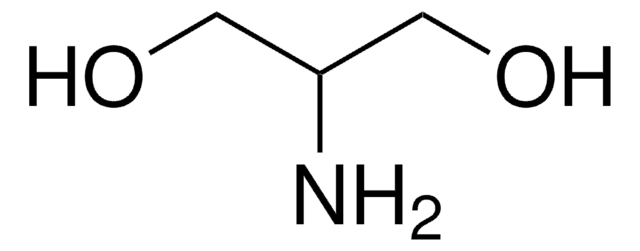
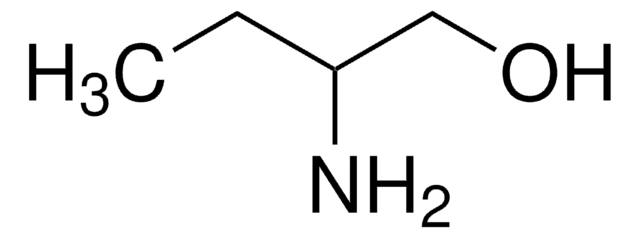
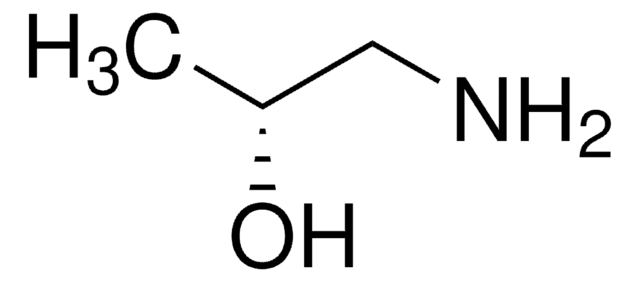
DL-Alaninol
98%
Synonym(s):
(±)-2-Amino-1-propanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH(NH2)CH2OH
CAS Number:
Molecular Weight:
75.11
Beilstein:
1209234
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
optical activity
[α]/D +1.0 to −1.0°
refractive index
n20/D 1.450 (lit.)
bp
173-176 °C (lit.)
density
0.943 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
SMILES string
CC(N)CO
InChI
1S/C3H9NO/c1-3(4)2-5/h3,5H,2,4H2,1H3
InChI key
BKMMTJMQCTUHRP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
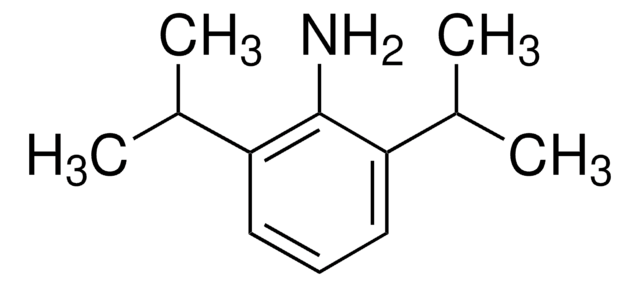
DL-Alaninol has been used in the synthesis of:
- N6-α-(I-hydroxypropyl) lysine
- diastereomers of DL-β-amino alcohols
- enantiopure and racemic samples of 2-methyl-N-tosylaziridine
- (±)-3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
183.2 °F - closed cup
Flash Point(C)
84 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
P M Hoi et al.
British journal of pharmacology, 152(5), 751-764 (2007-09-25)
A putative novel cannabinoid receptor mediates vasorelaxation to anandamide and abnormal-cannabidiol and is blocked by O-1918 and by high concentrations of rimonabant. This study investigates VSN16, a novel water-soluble agonist, as a vasorelaxant potentially acting at non-CB1, non-CB2 cannabinoid receptors
The role of lysine residues in the activity of 2-keto-3-deoxy-6-phosphogluconate aldolase.
J M Ingram et al.
The Journal of biological chemistry, 240(11), 4146-4151 (1965-11-01)
Ian C Stewart et al.
Journal of the American Chemical Society, 127(50), 17616-17617 (2005-12-15)
Sulfonylaziridines have been identified as excellent monomers for living ring-opening polymerization initiated by nucleophilic sulfonylamides. The resulting polymers exhibit low polydispersities and controllable molecular weights. The enantiopurity of the monomer plays a key role: racemic samples yield soluble polymers of
R LoBrutto et al.
Biochemistry, 40(1), 9-14 (2001-01-05)
The mechanism of propagation of the radical center between the cofactor, substrate, and product in the adenosylcobalamin- (AdoCbl) dependent reaction of ethanolamine ammonia-lyase has been probed by pulsed electron nuclear double resonance (ENDOR) spectroscopy. The radical of S-2-aminopropanol, which appears
Vahe Bandarian et al.
Biochemistry, 41(27), 8580-8588 (2002-07-03)
The structure of the steady-state radical intermediate in the deamination of S-2-aminopropanol catalyzed by ethanolamine ammonia-lyase (EAL) from Salmonella typhimurium has been probed by electron paramagnetic resonance (EPR) spectroscopy using isotopically labeled forms of the substrate and of the adenosylcobalamin
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service