187070
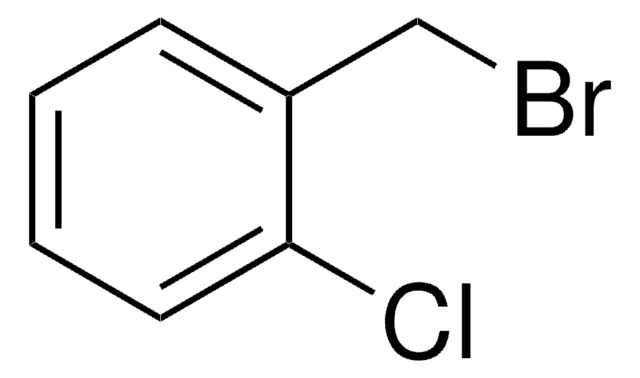
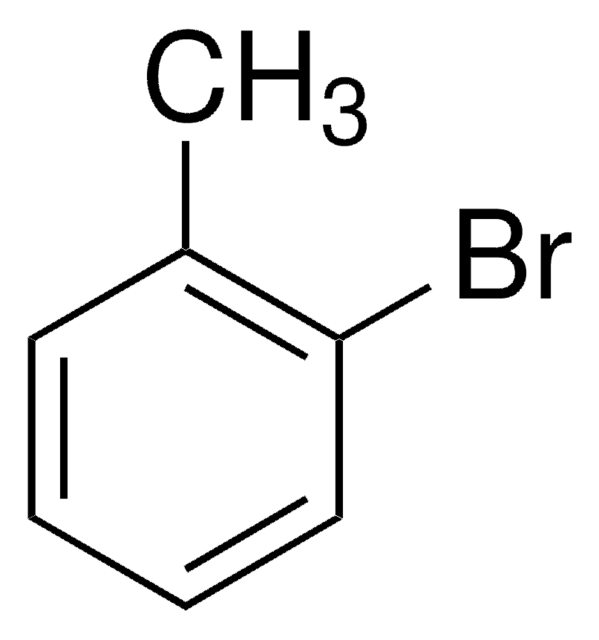
2-Bromobenzyl bromide
98%
Synonym(s):
α,2-Dibromotoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
BrC6H4CH2Br
CAS Number:
Molecular Weight:
249.93
Beilstein:
971015
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
refractive index
n20/D 1.619 (lit.)
bp
129 °C/19 mmHg (lit.)
mp
29-32 °C (lit.)
solubility
dioxane: soluble 1 g/10 mL, clear, colorless
functional group
bromo
SMILES string
BrCc1ccccc1Br
InChI
1S/C7H6Br2/c8-5-6-3-1-2-4-7(6)9/h1-4H,5H2
InChI key
LZSYGJNFCREHMD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Bromobenzyl bromide is a reagent used to protect ketones and aldehydes in their less reactive alcohol oxidation states and as a coupling component in various reactions.
Application
2-Bromobenzyl bromide was used in the synthesis of:
- substituted quinazolines and 1,2,3,4-tetrahydroquinazolines
- 2- and 3-substituted indenes
- tris-2-bromotribenzylamine
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and structural studies of tris-2-chlorobenzylamine and tris-2-bromobenzylamine.
Chen Q, , et al.
Journal of Chemical Crystallography, 35(3), 177-181 (2005)
Efficient synthesis of 2-and 3-substituted indenes from 2-bromobenzyl bromide through an enolate alkylation/Cr (II)/Ni (II)-mediated carbonyl addition sequence.
Halterman RLand Zhu C.
Tetrahedron Letters, 40(42), 7445-7448 (1999)
Xuesen Fan et al.
Chemistry, an Asian journal, 9(3), 739-743 (2014-01-01)
An efficient synthesis of diversely substituted quinazolines and 1,2,3,4-tetrahydroquinazolines through copper-catalyzed tandem reactions of the readily available 2-bromobenzyl bromides, aldehydes, and aqueous ammonia or amines has been developed. By using ammonia and simple aliphatic amines as the nitrogen source, the
Prachi Singh et al.
SLAS discovery : advancing life sciences R & D, 22(4), 440-446 (2017-03-23)
Analysis of interactions between molecules is of fundamental importance in life science research. In this study, we applied weak affinity chromatography, based on high-performance liquid chromatography and mass spectrometry, as a powerful tool for direct analysis of the components of
William L Scott et al.
Journal of combinatorial chemistry, 11(1), 14-33 (2008-12-25)
Distributed Drug Discovery (D(3)) proposes solving large drug discovery problems by breaking them into smaller units for processing at multiple sites. A key component of the synthetic and computational stages of D(3) is the global rehearsal of prospective reagents and
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 187070-25G | 4061838758408 |
| 187070-100G | 4061837594946 |
| 187070-5G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service