186279
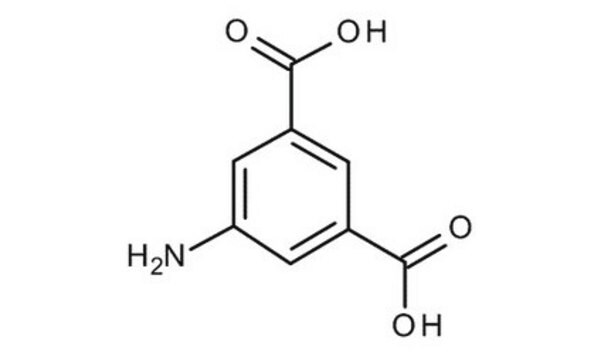
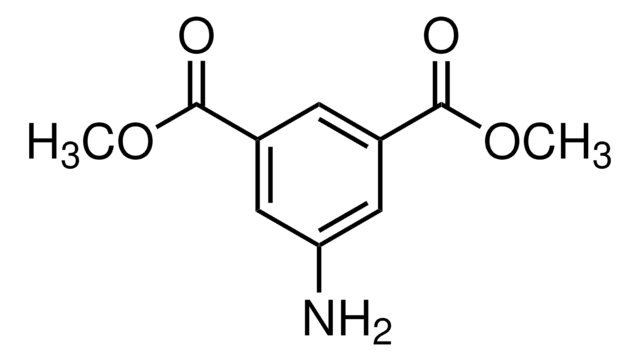
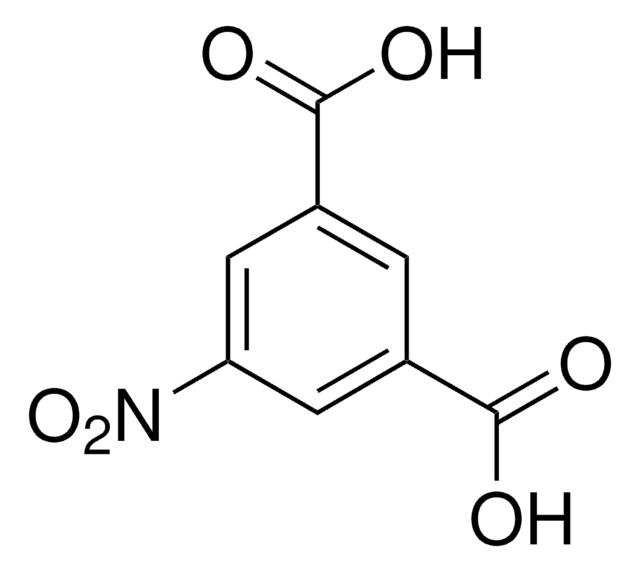
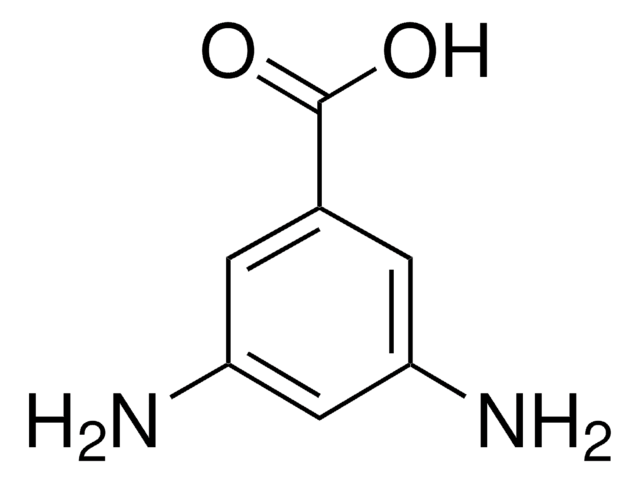
5-Aminoisophthalic acid
94%
Synonym(s):
5-Aminobenzene-1,3-dicarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H3-1,3-(CO2H)2
CAS Number:
Molecular Weight:
181.15
Beilstein:
2805628
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
94%
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
Nc1cc(cc(c1)C(O)=O)C(O)=O
InChI
1S/C8H7NO4/c9-6-2-4(7(10)11)1-5(3-6)8(12)13/h1-3H,9H2,(H,10,11)(H,12,13)
InChI key
KBZFDRWPMZESDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
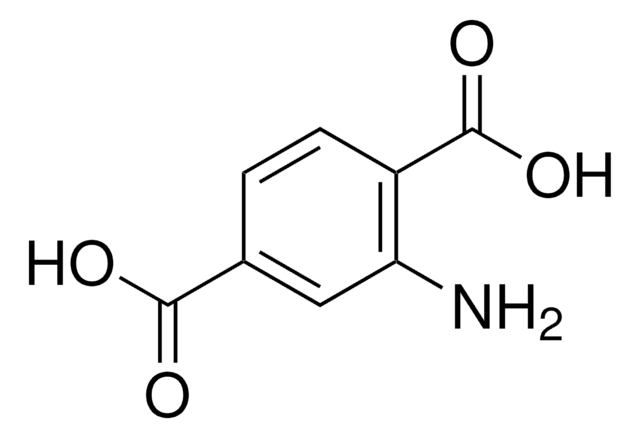
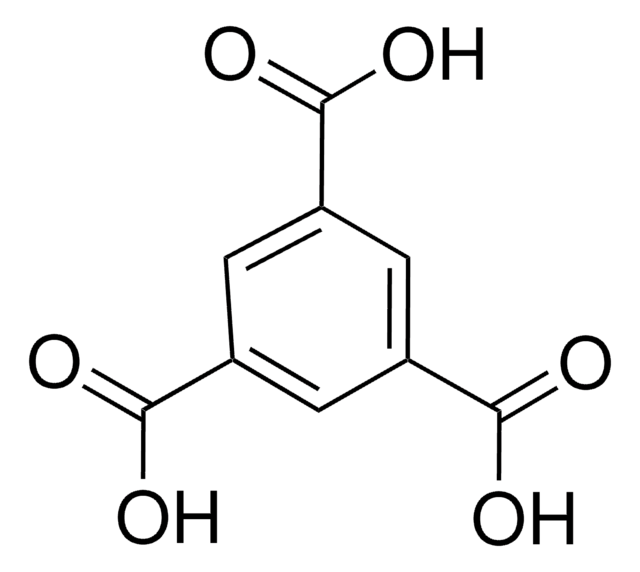
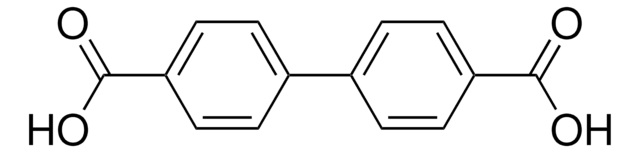
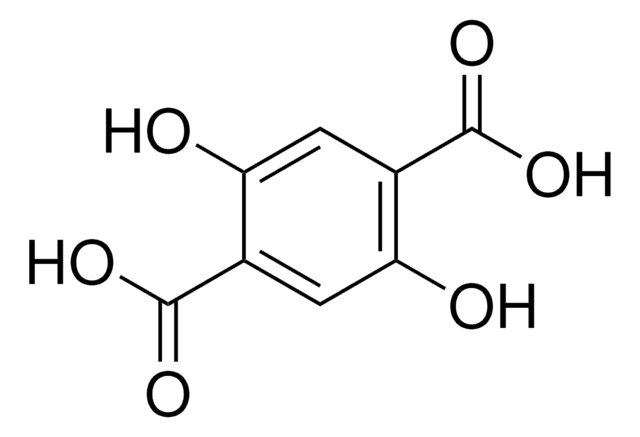
5-Aminoisophthalic acid (AIPA) can be used as a starting material to prepare:
It can also be used as a blended material with polyvinylalcohol to prepare silica-functionalized composite membranes for diffusion dialysis applications. AIPA based metal-organic framework (MOF) can be modified with various functional groups for different applications.
- Poly(5-aminoisophthalic acid) by oxidative polymerization reaction.
- Poly(benzimidazole-co-aniline) (PBIANI) by condensation polymerization with 3,3′-diaminobenzidine via poly(5-aminoisophthalic acid) as a key intermediate.
It can also be used as a blended material with polyvinylalcohol to prepare silica-functionalized composite membranes for diffusion dialysis applications. AIPA based metal-organic framework (MOF) can be modified with various functional groups for different applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S Crapatureanu et al.
Bioconjugate chemistry, 10(6), 1058-1067 (1999-11-24)
Hemoglobin can be cross-linked and converted to a bioconjugate in one step by a "molecular necklace", a reagent that contains two reacting sites and a pendant ligand. The compound to be conjugated is activated as an electrophile. The activated material
A Komersová et al.
Journal of pharmaceutical and biomedical analysis, 16(8), 1373-1379 (1998-10-20)
Application of differential-pulse cathodic stripping voltammetry using a carbon paste electrode (consisting of carbon powder and liquid paraffin) have been investigated for trace determination of iron in 5-aminoisophthalic acid (AIPA). Samples were dissolved in 1 M HC1, pH was adjusted
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 54 ( Pt 10), 1503-1505 (1998-11-10)
The title acid, C8H7NO4.0.5H2O, crystallized in the centrosymmetric space group C2/c in a zwitterionic form (5-ammonioisophthalate), with the water molecule on a twofold axis. The three ammonio H atoms and the H atom on the remaining carboxyl group, which are
A new self-cross-linked, net-structured, proton conducting polymer membrane for high temperature proton exchange membrane fuel cells
Bhadra S, et al.
Journal of Membrane Science , 349(1-2), 304-311 (2010)
Post-synthetic modification of a metal-organic framework based on 5-aminoisophthalic acid for mercury sorption
Xu Wei-Qin, et al.
Inorganic Chemistry Communications, 108(1-2), 107515-107515 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service