159549
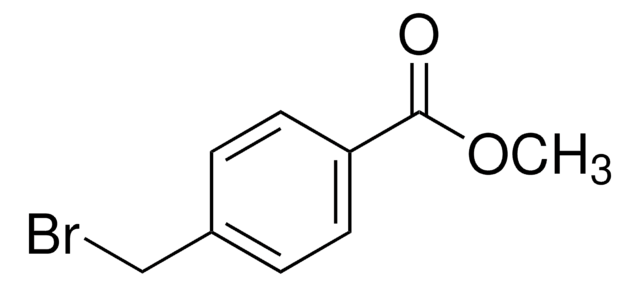
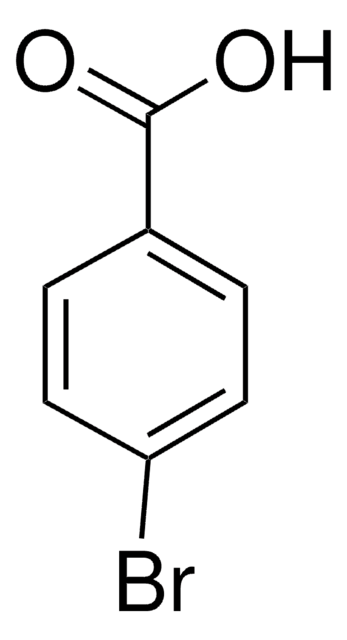
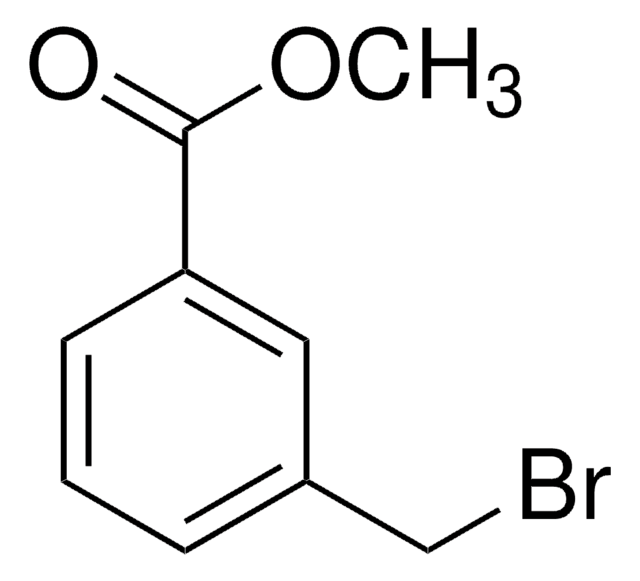
4-(Bromomethyl)benzoic acid
97%
Synonym(s):
α-Bromo-p-toluic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2C6H4CO2H
CAS Number:
Molecular Weight:
215.04
Beilstein:
1862870
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
224-229 °C (lit.)
functional group
bromo
carboxylic acid
SMILES string
OC(=O)c1ccc(CBr)cc1
InChI
1S/C8H7BrO2/c9-5-6-1-3-7(4-2-6)8(10)11/h1-4H,5H2,(H,10,11)
InChI key
CQQSQBRPAJSTFB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-(Bromomethyl)benzoic acid was used in the chemical modification of 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (temoporfin), second generation photosensitizer. It was also used in the synthesis of 4-(5-arylidene-2,4-dioxothiazolidin-3-yl) methylbenzoic acids.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Q Yu et al.
Chemical communications (Cambridge, England), 50(81), 12150-12153 (2014-09-02)
LiYF4:Tm(3+)/Yb(3+) upconverting nanoparticles (UCNPs) were functionalized with the second generation photosensitizer 5,10,15,20-tetra(m-hydroxyphenyl)chlorin (m-THPC, Temoporfin, Foscan®). m-THPC was modified using 4-(bromomethyl)benzoic acid, which induced a bathochromic shift of the m-THPC blue absorption peak. The nanoconstruct causes up to 70% cell death
Rosanna Maccari et al.
Bioorganic & medicinal chemistry, 15(15), 5137-5149 (2007-06-05)
4-(5-Arylidene-2,4-dioxothiazolidin-3-yl)methylbenzoic acids (2) were synthesized and evaluated in vitro as inhibitors of PTP1B and LMW-PTP, two protein tyrosine phosphatases (PTPs) which act as negative regulators of the metabolic and mitotic signalling of insulin. The synthesis of compounds 2 represents an
Marcela S Lopes et al.
European journal of medicinal chemistry, 46(11), 5443-5447 (2011-09-24)
A series of nitroaromatic compounds was synthesized and evaluated as potential antileishmanial and trypanocidal agents. Five compounds exerted significant anti-leishmanial activity in vitro against promastigotes forms of Leishmania (L.) amazonensis, with IC(50) in the range of 23-59 μmol L(-1), but
Salvatore Lombardo et al.
Langmuir : the ACS journal of surfaces and colloids, 33(22), 5473-5481 (2017-05-13)
The interaction of bovine serum albumin (BSA) with sulfated, carboxylated, and pyridinium-grafted cellulose nanocrystals (CNCs) was studied as a function of the degree of substitution by determining the adsorption isotherm and by directly measuring the thermodynamics of interaction. The adsorption
Zhimei He et al.
Small (Weinheim an der Bergstrasse, Germany), 15(4), e1804131-e1804131 (2018-12-20)
During photodynamic therapy (PDT), severe hypoxia often occurs as an undesirable limitation of PDT owing to the O2 -consuming photodynamic process, compromising the effectiveness of PDT. To overcome this problem, several strategies aiming to improve tumor oxygenation are developed. Unlike
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service