109622
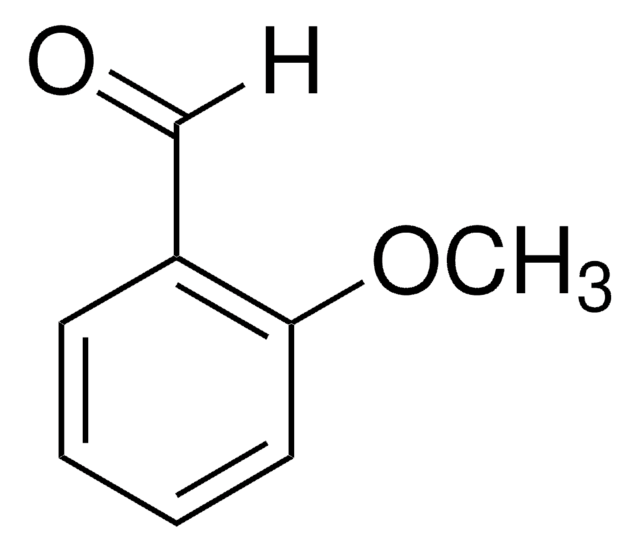
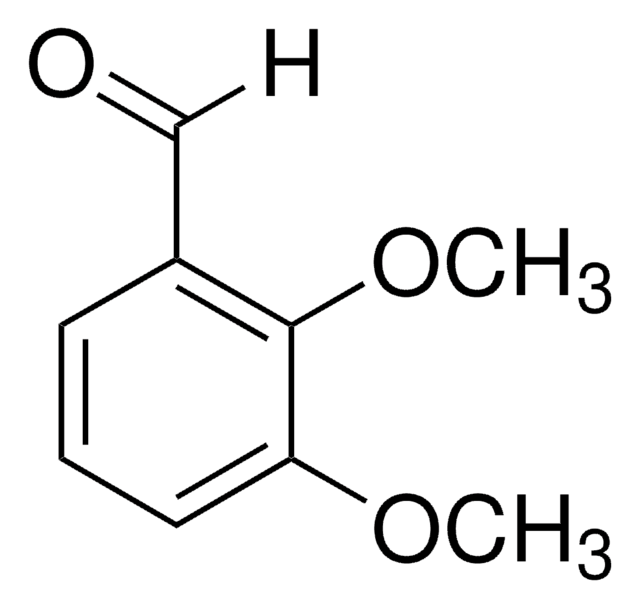
2-Methoxybenzaldehyde
98%
Synonym(s):
o-Anisaldehyde, Salicylaldehyde methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H4CHO
CAS Number:
Molecular Weight:
136.15
Beilstein:
606301
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
bp
238 °C (lit.)
mp
34-40 °C (lit.)
density
1.127 g/mL at 25 °C (lit.)
functional group
aldehyde
SMILES string
[H]C(=O)c1ccccc1OC
InChI
1S/C8H8O2/c1-10-8-5-3-2-4-7(8)6-9/h2-6H,1H3
InChI key
PKZJLOCLABXVMC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Methoxybenzaldehyde is used as a starting material to synthesize corrosion-inhibiting Schiff bases. Also used as a flavor and fragrance ingredient.
Application
2-Methoxybenzaldehyde has been used to examine the acaricidal activity of Periploca sepium oil and its active component against Tyrophagus putrescentiae.
2-Methoxybenzaldehyde has been used to obtain good enantioselectivities using Cu(OAc)(2)-bis(oxazolines) via hydrogen bonding in asymmetric Henry reaction.
Biochem/physiol Actions
Naturally-occurring aromatic aldehyde with acaricidal activity. May condense with L-tryptophan in foods to form the corresponding phenolic tetrahydro-β-carboline-3-carboxylic acid which is a potent antioxidant.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
244.4 °F - closed cup
Flash Point(C)
118 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Eun-Young Jeong et al.
Journal of food protection, 75(1), 118-122 (2012-01-10)
The aim of this study was to examine the acaricidal activity of Periploca sepium oil and its active component against Tyrophagus putrescentiae. Based on its 50% lethal dose (LD(50) ) value, P. sepium oil (8.45 μg/cm(2)) was highly active against
Ninghua Yin et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 153, 1-5 (2015-08-19)
A facile fluorescence switch with Schiff base units was designed and achieved by nucleophilic addition and dehydration reaction. The fluorescence of the probe can be regulated by metal ions (Al(3+) and Cu(2+)). The whole process shows that the weak fluorescence
Olga V Kupriyanova et al.
Drug testing and analysis, 12(8), 1154-1170 (2020-05-18)
N-(2-Methoxybenzyl)-2,5-dimethoxyphenethylamines (NBOMes) are synthetic phenethylamine derivatives emerging on the global drug market and reported to be associated with untoward effects in people who use drugs. Its action involves agonism at serotonin 5-HT2A receptors, affecting cognitive and behavioral processes. However, certain
Zhi-Huai Li et al.
Chirality, 24(12), 1092-1095 (2012-09-25)
Immobilized Cu(OAc)(2)-bis(oxazolines) via hydrogen bonding by SBA-15 was applied to asymmetric Henry reaction, and good enantioselectivities were obtained (up to 83% ee) between 2-methoxybenzaldehyde and CH(3)NO(2) in isopropyl alcohol (iPrOH). The catalyst could be reused seven times without any obvious
Chao-Bin Xue et al.
Bioorganic & medicinal chemistry, 15(5), 2006-2015 (2007-01-30)
Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-quinones. Inhibition of PO may provide a basis for novel environmentally friendly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service