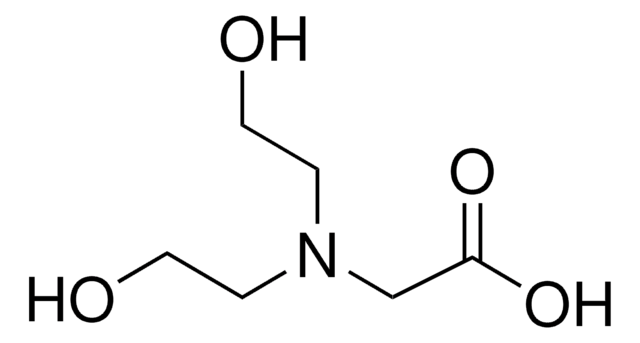
T9269
TAPSO
≥99% (titration)
Synonym(s):
2-Hydroxy-3-[tris(hydroxymethyl)methylamino]-1-propanesulfonic acid, N-[Tris(hydroxymethyl)methyl]-3-amino-2-hydroxypropanesulfonic acid
About This Item
Recommended Products
Quality Level
Assay
≥99% (titration)
form
crystalline powder
useful pH range
7.0-8.2
pKa (25 °C)
7.6
solubility
warm water: 0.333 g/mL, clear to slightly hazy, colorless
application(s)
diagnostic assay manufacturing
SMILES string
OCC(CO)(CO)NCC(O)CS(O)(=O)=O
InChI
1S/C7H17NO7S/c9-3-7(4-10,5-11)8-1-6(12)2-16(13,14)15/h6,8-12H,1-5H2,(H,13,14,15)
InChI key
RZQXOGQSPBYUKH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Interpretation of non-Nernstian slopes in graphic analysis of data collected in pH range close to deprotonation of a ligand Part I. A glass electrode potentiometric and polarographic study of Cd-(TAPSO)x-(OH)y and Zn-(TAPSO)x-(OH)y systems.: Explores the complex behavior of TAPSO in metal binding studies, particularly with cadmium and zinc, providing insight into its utility in heavy metal chelation and environmental cleanup strategies (Machado et al., 2006).
- Challenges in modelling and optimisation of stability constants in the study of Cu-(TAPS)(x)-(OH)(y) system by polarography.: Discusses the challenges in modeling and optimizing the stability constants for copper complexes with TAPSO, contributing to better understanding and utilization of this buffer in copper-related biochemical research (Machado and Soares, 2007).
- Modelling of Pb-(TAPS)(x)-(OH)(y) system and refinement of stability constants in the region of lead hydrolysis and lead hydroxide precipitation.: Provides a detailed examination of TAPSO′s role in modeling lead complexation systems, which is critical for environmental monitoring and remediation efforts involving lead (Machado et al., 2007).
- Synthetic organic pH buffers can support fertilization of guinea pig eggs, but not as efficiently as bicarbonate buffer.: Evaluates the efficacy of synthetic buffers like TAPSO in supporting fertilization, offering valuable data for reproductive biology and assisted reproductive technologies (Bhattacharyya and Yanagimachi, 1988).
Caution
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service