S2563
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp.
Synonym(s):
SCDase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
foreign activity
exoglycosidases: α- and β-galactosidase, α- and β-N-acetylgalactosaminidase, β-N-acetylglucosaminidase, α-mannosidase, α-fucosidase, and sialidase., essentially free
protease and sphingomyelinase, essentially free
Quality Level
shipped in
dry ice
storage temp.
−20°C
General description
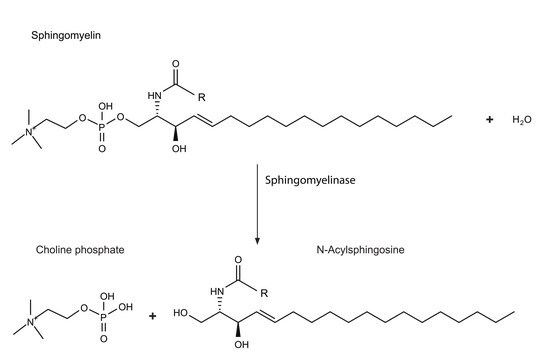
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp. is a hydrolytic enzyme.
Application
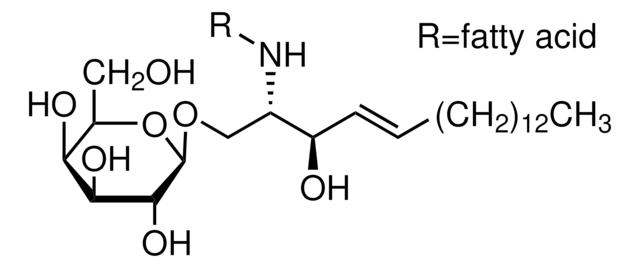
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp. has been used in enzymatic conversion and derivatization of sulfatides. It has also been used to hydrolyze the fatty acid chain of mactosyl ceramide.
Biochem/physiol Actions
Sphingolipid Ceramide N-Deacylase from Pseudomonas sp., under certain conditions, can recyclate lyso-sphingolipids. It condenses fatty acids to sphingosine to generate ceramide. The enzyme mainly acts on neutral and acidic glycosphingolipids.
Hydrolyzes the N-acyl linkage between fatty acids and sphingosines in ceramides of various sphingolipids.
Unit Definition
One unit will hydrolyze 1 μmol of asialo GM1 per minute at pH 6.0 at 37 °C.
Physical form
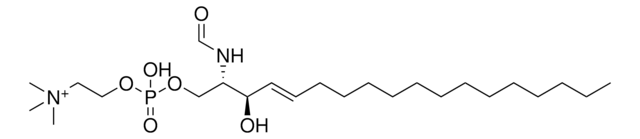
Solution in 50 mM sodium acetate, pH 6.0, with 0.1% Lubrol PX.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Characterization of the reversible nature of the reaction catalyzed by sphingolipid ceramide N-deacylase. A novel form of reverse hydrolysis reaction.
Kita K
European Journal of Biochemistry, 268(3), 592-602 (2001)
Sulfatide Analysis by Mass Spectrometry for Screening of Metachromatic Leukodystrophy in Dried Blood and Urine Samples.
Spacil Z
Clinical Chemistry, 62(1), 279-286 (2016)
A novel enzyme that cleaves the N-acyl linkage of ceramides in various glycosphingolipids as well as sphingomyelin to produce their lyso forms.
Ito M
The Journal of Biological Chemistry, 270(41), 24370-24374 (1995)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service