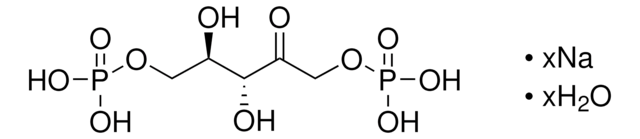
R0878
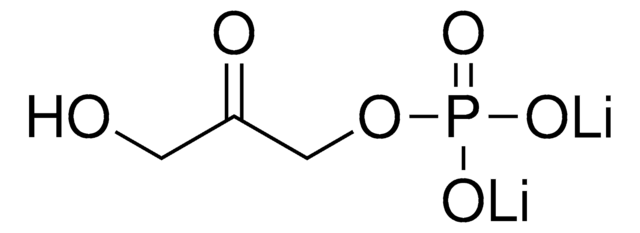
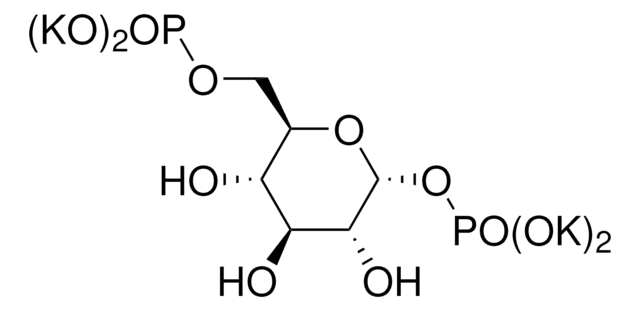
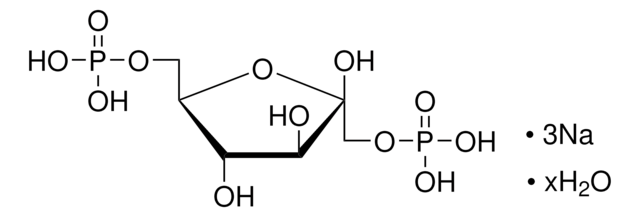
D-Ribulose 1,5-bisphosphate sodium salt hydrate
≥90% (TLC)
Synonym(s):
D-erythro-2-Pentulose, 1,5-bis(dihydrogen phosphate), D-Ribulose 1,5-diphosphate, RuDP
About This Item
Recommended Products
biological source
synthetic (inorganic)
Assay
≥90% (TLC)
form
powder
impurities
≤16% water (Karl Fischer)
color
white
solubility
water: ~50 g/L
cation traces
Na: 17.1-24.6% (dry basis)
storage temp.
−20°C
SMILES string
[P](=O)(OC[C@@H](O)[C@@H](O)C(=O)CO[P](=O)(O)O)(O)O
InChI
1S/C5H12O11P2/c6-3(1-15-17(9,10)11)5(8)4(7)2-16-18(12,13)14/h3,5-6,8H,1-2H2,(H2,9,10,11)(H2,12,13,14)/t3-,5-/m1/s1
InChI key
YAHZABJORDUQGO-NQXXGFSBSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Bio-Inspired Microreactors Continuously Synthesize Glucose Precursor from CO(2) with an Energy Conversion Efficiency 3.3 Times of Rice.: This study leverages D-Ribulose 1,5-bisphosphate sodium salt hydrate in bio-inspired microreactors to synthesize glucose precursors from CO₂. The approach demonstrates an energy conversion efficiency significantly higher than that of rice, highlighting its potential for efficient carbon fixation and bioengineering applications (Zhu et al., 2024).
- Designing Stacked Assembly of Type III Rubisco for CO₂ Fixation with Higher Efficiency.: The research utilizes D-Ribulose 1,5-bisphosphate sodium salt hydrate to enhance the efficiency of CO₂ fixation through the stacked assembly of Type III Rubisco. This innovative approach offers improvements in metabolic engineering for carbon capture and utilization (Zeng et al., 2022).
- Continuous artificial synthesis of glucose precursor using enzyme-immobilized microfluidic reactors.: This study explores the continuous synthesis of glucose precursors using microfluidic reactors with immobilized enzymes, including D-Ribulose 1,5-bisphosphate sodium salt hydrate. The technique demonstrates potential for scalable biochemical production processes (Zhu et al., 2019).
Biochem/physiol Actions
Other Notes
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service