G6657
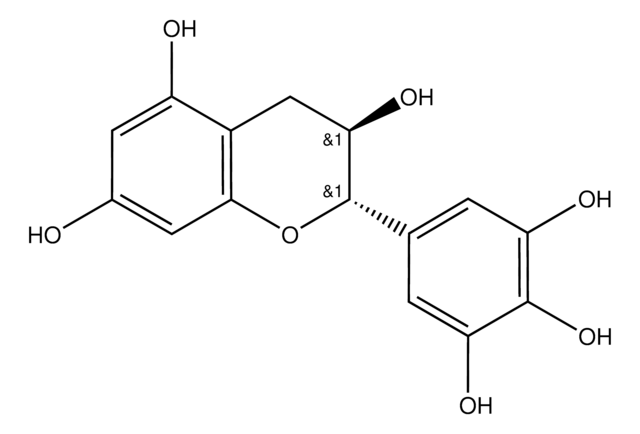
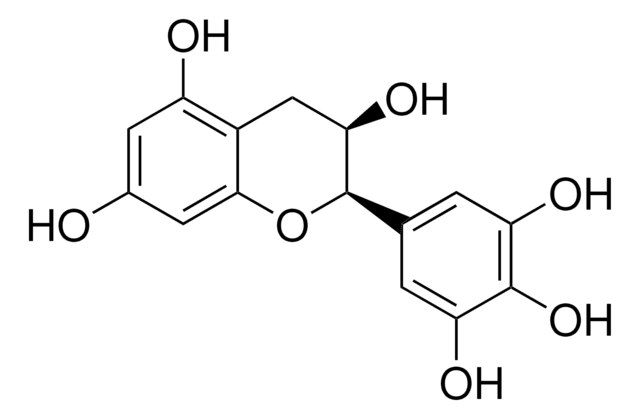
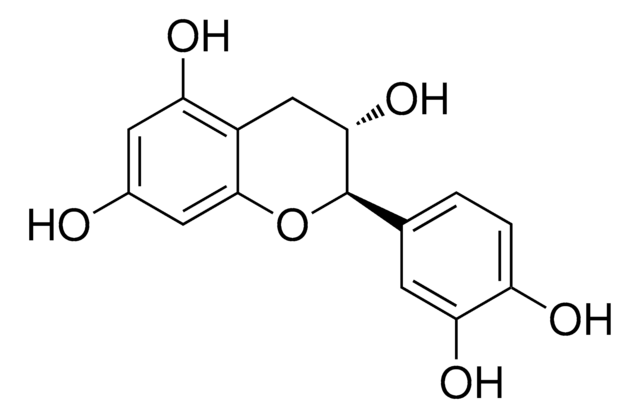
(−)-Gallocatechin
≥98% (HPLC)
Synonym(s):
(2S,3R)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
solubility
H2O: 5 mg/mL (heat 2-10 min at 105C)
alcohol: soluble
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
O[C@@H]1Cc2c(O)cc(O)cc2O[C@H]1c3cc(O)c(O)c(O)c3
InChI
1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15+/m1/s1
InChI key
XMOCLSLCDHWDHP-DOMZBBRYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- for the identification of phenolic compounds present in Pineapple (Ananas comosus) using high-performance liquid chromatography (HPLC)
- to evaluate the catechin profiles from Camellia sinensis green tea, white tea, and flower sample by high-performance liquid chromatography/photodiode array detection (RP-HPLC/PDAD) analysis
- as a reference standard for the flavan-3-ols profiling muscadine grape hybrid varieties using high-performance liquid chromatography-quadrupole, time-of-flight, tandem mass spectrometry (HPLC-qTOF-MS/MS) analysis
Biochem/physiol Actions
Other Notes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service