G5001
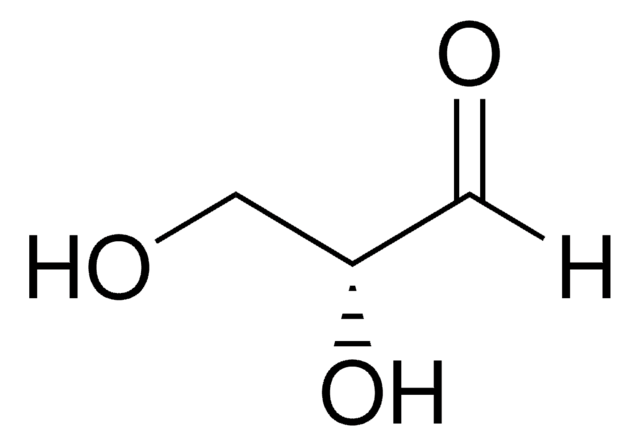
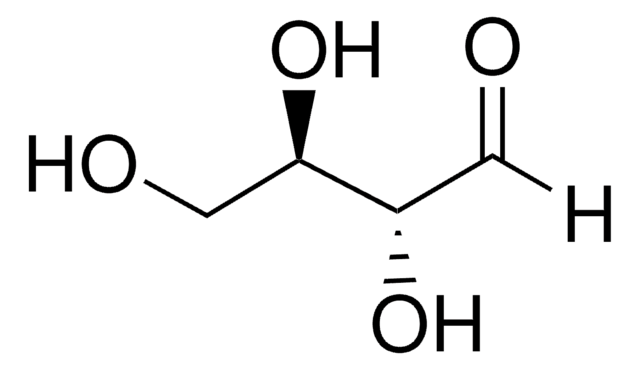
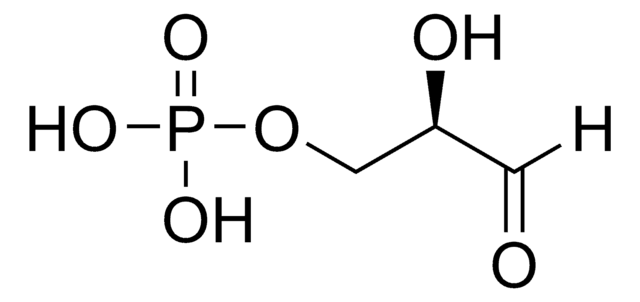
DL-Glyceraldehyde
≥90% (GC)
Synonym(s):
α,β-Dihydroxypropionaldehyde, 2,3-Dihydroxypropanal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
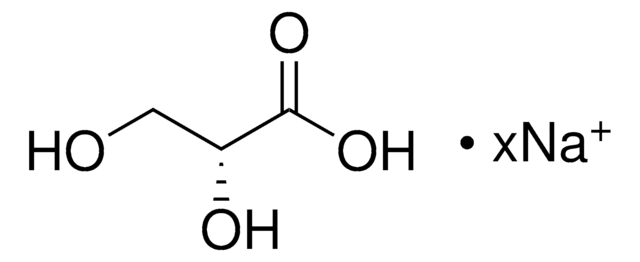
C3H6O3
CAS Number:
Molecular Weight:
90.08
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.25
Recommended Products
biological source
synthetic
Quality Level
Assay
≥90% (GC)
form
powder
color
white to off-white
mp
145 °C ((293 °F ))
solubility
water: 50 mg/mL, clear to slightly hazy, colorless to faintly yellow
storage temp.
room temp
SMILES string
[H]C(=O)C(O)CO
InChI
1S/C3H6O3/c4-1-3(6)2-5/h1,3,5-6H,2H2
InChI key
MNQZXJOMYWMBOU-UHFFFAOYSA-N
General description
Glyceraldehyde is a simple monosaccharide. Based on the number of carbon atoms and the type of carbonyl group present, glyceraldehyde belongs to subgroup triose. It is a colourless and sweet compound.
Application
DL-Glyceraldehyde has been used:
- as modifying reagent in the preparation of crystallization solution
- as a substrate to measure aldose reductase activity
- in the preparation of d/l-glyceraldehyde stock to determine the specific activity of GAPDH (glyceraldehyde 3-phosphate dehydrogenase)
Biochem/physiol Actions
Glyceraldehyde serves as an efficient cross-linking agent and is considered non-toxic. It is an intermediate of a number of metabolic such as glycolysis and pentose phosphate pathway.
Other Notes
DL-Glyceraldehyde is a substrate for the enzyme aldose reductase.
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gemma Sangüesa et al.
European journal of nutrition, 58(3), 1283-1297 (2018-03-09)
Sugar-sweetened beverage intake is a risk factor for insulin resistance, dyslipidemia, fatty liver, and steatohepatitis (NASH). Sub-chronic supplementation of liquid fructose, but not glucose, in female rats increases liver and plasma triglycerides without inflammation. We hypothesized that chronic supplementation of
Raman and infrared spectroscopy of carbohydrates: a review
Wiercigroch E, et al.
Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy, 185(5), 317-335 (2017)
Chethan Sampath et al.
Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie, 84, 502-513 (2016-09-30)
Hyperglycemic stress activates polyol pathway and aldose reductase (AR) key enzyme responsible for generating secondary complications during diabetes. In this study the therapeutic potential of phloretin, epigallocatechin 3-gallate (EGCG) and [6]-gingerol were evaluated for anti-glycating and AR inhibitory activity in
GAPDH and Intermediary Metabolism
GAPDH: Biological Properties and Diversity, 37-59 (2012)
Stabilization of scleral collagen by glycerol aldehyde cross-linking
N.A.Danilov, et al.
Biochimica et Biophysica Acta, 1780(5), 764-772 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service