F7250
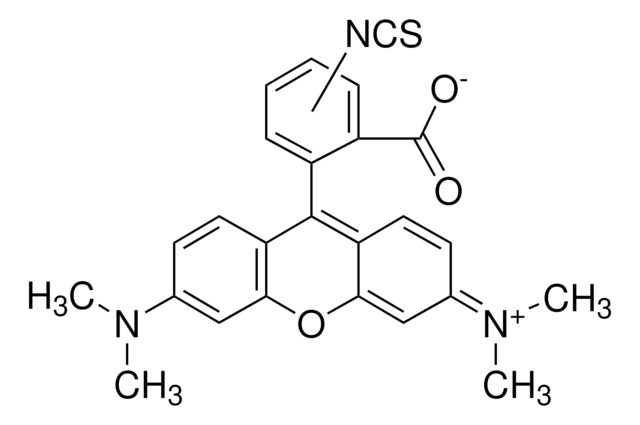
Fluorescein isothiocyanate isomer I
≥90% purity (HPLC), powder
Synonym(s):
FITC, Fluorescein 5-isothiocyanate
About This Item
Recommended Products
Product Name
Fluorescein isothiocyanate isomer I, suitable for protein labeling, ≥90% (HPLC), powder
Assay
≥90% (HPLC)
form
powder
technique(s)
titration: suitable
color
orange to dark orange
mp
>360 °C (lit.)
solubility
acetone: 1 mg/mL
fluorescence
λex 492 nm; λem 518 nm (green)
suitability
suitable for protein labeling
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
2-8°C
SMILES string
Oc1ccc2c(Oc3cc(O)ccc3C24OC(=O)c5cc(ccc45)N=C=S)c1
InChI
1S/C21H11NO5S/c23-12-2-5-16-18(8-12)26-19-9-13(24)3-6-17(19)21(16)15-4-1-11(22-10-28)7-14(15)20(25)27-21/h1-9,23-24H
InChI key
MHMNJMPURVTYEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
It is widely used to attach a fluorescent label to proteins via the amine group. The isothiocyanate group reacts with amino terminal and primary amines in proteins. It has been used for the labeling of proteins including antibodies and lectins.
Fluorescein isothiocyanate isomer I has been proposed as a contact sensitizer.
Application
Biological applications include use as a fluorescent labeling reagent for proteins, a fluorescent reagent for protein tracing, and a reagent in the fluorescent antibody technique for the rapid identification of pathogens. It may be employed as the derivatization reagent for amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine and P-phenylethylamine in human urine during their capillary electrophoretic (CE) determination. It may be used for the preparation of fluorescent antibodies. It was employed for in vitro sensitization studies.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service