D2821
Deoxyribonuclease I bovine
recombinant, expressed in Pichia pastoris, lyophilized powder, RNAse and protease, free
Synonym(s):
DNAse I, Deoxyribonucleate 5′-oligonucleotido-hydrolase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
bovine
Quality Level
recombinant
expressed in Pichia pastoris
form
lyophilized powder
specific activity
≥4,000 units/mg protein
mol wt
~39 kDa
technique(s)
DNA extraction: suitable
solubility
H2O: soluble (pH 4.0-9.0)
suitability
suitable for molecular biology
application(s)
diagnostic assay manufacturing
foreign activity
RNAse and protease, free
storage temp.
2-8°C
Looking for similar products? Visit Product Comparison Guide
Application
Deoxyribonuclease I bovine has been used in a study to investigate the inhibition of DNA polymerase by extracts of rat liver. Deoxyribonuclease I bovine has also been used in a study to investigate the effects of ionic strength on enzymic activity.
Deoxyribonuclease I bovine has been used in the preparation of cold cell lysis buffer, complete RNA lysis buffer, cell lysis buffer for testing the expression of recombinant tagged protein.
The enzyme from Sigma has been used for the digestion of DNA extracted from Agave spp. clones. The enzyme was used during a study that investigated the effect of epigenetic changes on the regulatory expression of KNOTTED1-like HOMEOBOX (KNOX) transcription factors.
Used for the removal of DNA from protein samples.
Biochem/physiol Actions
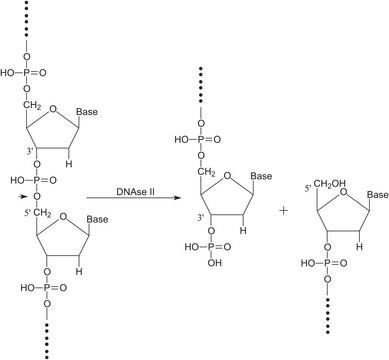
DNase I is an endonuclease that acts on phosphodiester bonds adjacent to pyrimidines to produce polynucleotides with terminal 5′-phosphates. The pH optimum is found to be between 7 and 8. Divalent cations such as Mn2+, Ca2+, Co2+, and Zn2+ are activators of the enzyme. A concentration of 5 mM Ca2+ stabilizes the enzyme against proteolytic digestion. 2-Mercaptoethanol, chelators, sodium dodecyl sulfate (SDS) and actin are known to inhibit the enzyme activity.
Digests single- and double-stranded DNA to a mixture of mono- and oligonucleotides carrying 5′ phosphates and 3′ OH termini. This catalytic activity is divalent ion-dependent. In the presence of Mg2+, DNase I hydrolyzes each strand of double-stranded DNA randomly and independently. In the presence of Mn2+, both strands can be cleaved.
Features and Benefits
- RNA purification by removing DNA
- Prepare DNA for nick translation1
- Footprinting assays to determine DNA-protein interactions2
Unit Definition
One unit will produce a ΔA260 of 0.001 per min per mL reaction mixture using calf thymus DNA at pH 5.0 and 25°C
Physical form
supplied as a lyophilized powder containing glycine as a stabilizer
Preparation Note
Produced without using any animal cells or animal derived materials.
The enzyme powder may be reconstituted in water or any buffer at pH 4.0-9.0, except phosphate buffer. Calcium chelators should be avoided. 10 mg/mL solution of DNAse I in 0.15 M NaCl may lose <10% of its activity when stored for a week in aliquots at –20 °C. The same solutions stored in aliquots at 2-8 °C can lose approximately 20% activity. It remains active for upto five hours at 60 °C and loses activity in <10 minutes at 68 °C. It loses activity at the rate of 6%/hour in acetate buffer (pH 5.0) and tris buffer ((pH 7.2) at 1 mg/mL concentration.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Recombinant expression and purification of an ATP-dependent DNA ligase from Aliivibrio salmonicida.
Williamson A and Pedersen H
Protein Expression and Purification, 97(4), 29-36 (2014)
Quantitative proteomics of breast tumors: Tissue quality assessment to clinical biomarkers.
Chen Y, et al.
Proteomics, 17(6), 1600335-1600335 (2017)
Genome-scale CRISPR-Cas9 knockout and transcriptional activation screening.
Joung J, et al.
Nature Protocols, 12(4), 828-828 (2017)
Adele Williamson et al.
Protein expression and purification, 97, 29-36 (2014-03-04)
The genome of the psychrophilic fish-pathogen Aliivibrio salmonicida encodes a putative ATP-dependent DNA ligase in addition to a housekeeping NAD-dependent enzyme. In order to study the structure and activity of the ATP dependent ligase in vitro we have undertaken its
Properties of chromatographically purified bovine pancreatic deoxyribonuclease.
P A Price et al.
The Journal of biological chemistry, 244(3), 917-923 (1969-02-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service