T28002
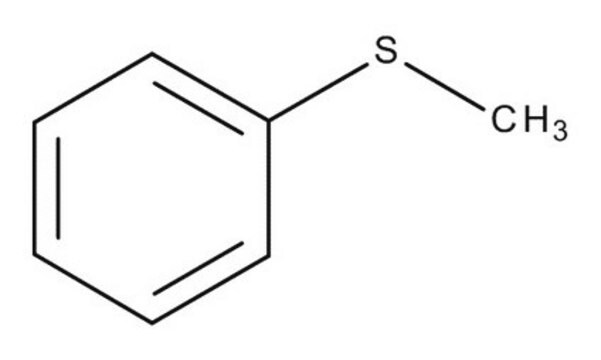
Thioanisole
ReagentPlus®, ≥99%
Synonym(s):
Methyl phenyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5SCH3
CAS Number:
Molecular Weight:
124.20
Beilstein:
1904179
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99%
refractive index
n20/D 1.587 (lit.)
bp
188 °C (lit.)
mp
−15 °C (lit.)
density
1.057 g/mL at 20 °C (lit.)
functional group
thioether
SMILES string
CSc1ccccc1
InChI
1S/C7H8S/c1-8-7-5-3-2-4-6-7/h2-6H,1H3
InChI key
HNKJADCVZUBCPG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
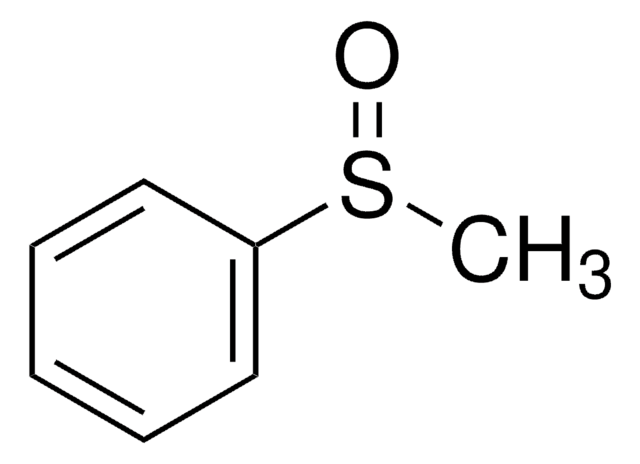
Thioanisole may be used in the synthesis of methyl phenyl sulfoxide via oxidation.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
163.4 °F - closed cup
Flash Point(C)
73 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Methyl phenyl sulfoxide.
Johnson CR & Keiser JE.
Organic Syntheses, 78-78 (1966)
Mild and selective oxidation of sulfur compounds in trifluoroethanol: diphenyl disulfide and methyl phenyl sulfoxide.
Ravikumar KS, et al.
Organic Syntheses, 184-189 (2003)
Rémy Ricoux et al.
Organic & biomolecular chemistry, 7(16), 3208-3211 (2009-07-31)
Two new artificial hemoproteins or "hemozymes", obtained by non covalent insertion of Fe(III)-meso-tetra-p-carboxy- and -p-sulfonato-phenylporphyrin into xylanase A from Streptomyces lividans, were characterized by UV-visible spectroscopy and molecular modeling studies, and were found to catalyze the chemo- and stereoselective oxidation
Jiyun Park et al.
Journal of the American Chemical Society, 133(14), 5236-5239 (2011-03-18)
The mechanism of sulfoxidation of thioaniosoles by a non-heme iron(IV)-oxo complex is switched from direct oxygen transfer to metal ion-coupled electron transfer by the presence of Sc(3+). The switch in the sulfoxidation mechanism is dependent on the one-electron oxidation potentials
Jun-Long Zhang et al.
Chemical communications (Cambridge, England), (14)(14), 1665-1667 (2008-03-28)
We demonstrate that incorporation of MnSalen into a protein scaffold enhances the chemoselectivity in sulfoxidation of thioanisole and find that both the polarity and hydrogen bonding of the protein scaffold play an important role in tuning the chemoselectivity.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service