860830P
Avanti
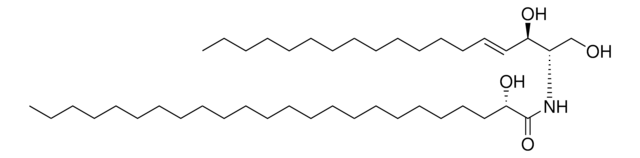
18:0(2S-OH) Ceramide
Avanti Research™ - A Croda Brand 860830P, powder
Synonym(s):
N-(2′-(S)-hydroxystearoyl)-D-erythro-sphingosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C36H71NO4
CAS Number:
Molecular Weight:
581.95
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
form
powder
packaging
pkg of 1 × 5 mg (860830P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 860830P
lipid type
sphingolipids
shipped in
dry ice
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCC/C=C/[C@@H](O)[C@@H](NC([C@@H](O)CCCCCCCCCCCCCCCC)=O)CO
General description
18:0(2S-OH) Ceramide is a hydroxy fatty acid (hFA)-sphingolipid containing 18C long chain base fatty acid (stearic acid)-with 2′-hydroxyl group in S configuration.
Biochem/physiol Actions
Hydroxy fatty acid containing ceramides are involved in epidermal permeability barrier function. 18:0(2S-OH) ceramide is vital for maintaining membrane fluidity.
Packaging
5 mL Amber Glass Screw Cap Vial (860830P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jennifer E Kyle et al.
The Analyst, 141(5), 1649-1659 (2016-01-07)
Understanding how biological molecules are generated, metabolized and eliminated in living systems is important for interpreting processes such as immune response and disease pathology. While genomic and proteomic studies have provided vast amounts of information over the last several decades
Ana B Herrero et al.
Cancer research, 68(23), 9779-9787 (2008-12-03)
PM02734 is a novel synthetic antitumor drug that is currently in phase I clinical trials. To gain some insight into its mode of action, we used the yeast Saccharomyces cerevisiae as a model system. Treatment of S. cerevisiae with PM02734
Nathan L Alderson et al.
Journal of lipid research, 50(6), 1203-1208 (2009-01-28)
Sphingolipids are ubiquitous components of eukaryotic cells that regulate various cellular functions. In many cell types, a fraction of sphingolipids contain 2-hydroxy fatty acids, produced by fatty acid 2-hydroxylase (FA2H), as the N-acyl chain of ceramide [hydroxyl fatty acid (hFA)-sphingolipids].
Hiroko Hama
Biochimica et biophysica acta, 1801(4), 405-414 (2009-12-23)
2-Hydroxy fatty acids (hFA) are important components of a subset of mammalian sphingolipids. The presence of hFA in sphingolipids is best described in the nervous system, epidermis, and kidney. However, the literature also indicates that various hFA-sphingolipids are present in
Zdzislaw M Szulc et al.
Bioorganic & medicinal chemistry, 18(21), 7565-7579 (2010-09-21)
A straightforward method for the simultaneous preparation of (2S,3R,2'R)- and (2S,3R,2'S)-2'-hydroxy-ceramides (2'-OHCer) from (2S,3R)-sphingosine acetonide precursors and racemic mixtures of 2-hydroxy fatty acids (2-OHFAs) is described. The obtained 2'-OH-C4-, -C6-, -C12-, -C16-Cer and 2'-OH-C6-dhCer pairs of diastereoisomers were characterized thoroughly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service