810226P
Avanti
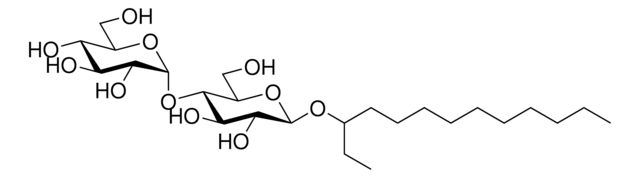
C6-NBD Lactosyl Ceramide
Avanti Research™ - A Croda Brand 810226P, powder
Synonym(s):
N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-D-lactosyl-β1-1′-sphingosine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C42H69N5O16
CAS Number:
Molecular Weight:
900.02
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 1 mg (810226P-1MG)
pkg of 1 × 250 μg (810226P-250ug)
manufacturer/tradename
Avanti Research™ - A Croda Brand 810226P
shipped in
dry ice
storage temp.
−20°C
General description
C6-NBD Lactosyl ceramide is a commercially available fluorescent analog of compound lactosyl ceramide. Lactosyl ceramide is present on neutrophils and macrophages.
Application
C6-NBD Lactosyl Ceramide has been used in:
- lactosylceramide synthase assay as a fluorescent acceptor substrate identified using fluorescence based detector in HPLC(1)
- phospholipid labeling and fluorescence-lifetime imaging microscopy (FILM) of cell membranes(6)
Biochem/physiol Actions
Lactosyl ceramide is utilized for synthesis of various glycosphingolipids like oligoglycosylceramides and gangliosides in vertebrates. It is an important molecule involved in signaling cascades leading to phenotypic changes such as adhesion, migration, cell proliferation and angiogenesis.
Packaging
5 mL Amber Glass Screw Cap Vial (810226P-1MG)
5 mL Amber Glass Screw Cap Vial (810226P-250ug)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
W I Weis et al.
Annual review of biochemistry, 65, 441-473 (1996-01-01)
Lectins are responsible for cell surface sugar recognition in bacteria, animals, and plants. Examples include bacterial toxins; animal receptors that mediate cell-cell interactions, uptake of glycoconjugates, and pathogen neutralization; and plant toxins and mitogens. The structural basis for selective sugar
S Hakomori et al.
Journal of biochemistry, 118(6), 1091-1103 (1995-12-01)
Glycosphingolipids (GSLs), cell type-specific markers which change dramatically during ontogenesis and oncogenesis, have been implicated as playing major roles in cellular interactions and control of cell proliferation in multicellular organisms. These functional roles have been partially clarified through two types
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service