W435600
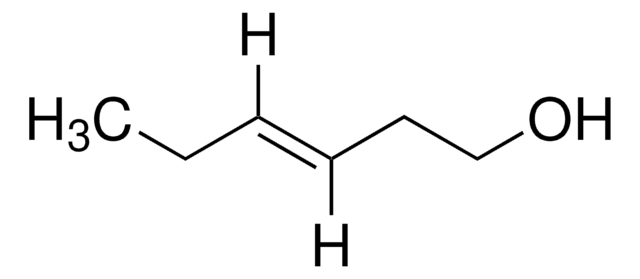
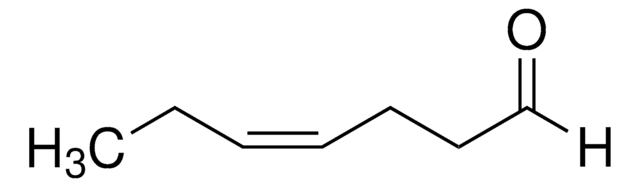
trans-3-Hexen-1-ol
≥95%, stabilized
Synonym(s):
trans-3-Hexenol
About This Item
Halal
Kosher
Recommended Products
biological source
synthetic
Quality Level
grade
Fragrance grade
Halal
Kosher
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
Assay
≥95%
contains
alpha-tocopherol, synthetic as stabilizer
refractive index
n20/D 1.439 (lit.)
bp
61-62 °C/12 mmHg (lit.)
density
0.817 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
Organoleptic
green
SMILES string
[H]\C(CC)=C(\[H])CCO
InChI
1S/C6H12O/c1-2-3-4-5-6-7/h3-4,7H,2,5-6H2,1H3/b4-3+
InChI key
UFLHIIWVXFIJGU-ONEGZZNKSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Disclaimer
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W435600-100G-K | 4061837862649 |
| W435600-1KG-K | 4061837862656 |
| W435600-SAMPLE-K | 4061837862663 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service