P46105
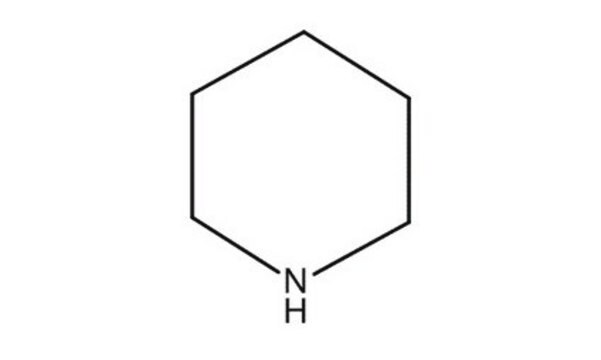
Piperidine hydrochloride
99%
Synonym(s):
Piperidinium chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11N · HCl
CAS Number:
Molecular Weight:
121.61
Beilstein:
3611699
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
2.94 (vs air)
Quality Level
vapor pressure
63.6 mmHg ( 37.8 °C)
Assay
99%
form
powder
mp
245-248 °C (lit.)
SMILES string
Cl[H].C1CCNCC1
InChI
1S/C5H11N.ClH/c1-2-4-6-5-3-1;/h6H,1-5H2;1H
InChI key
VEIWYFRREFUNRC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reagent for synthesis of:
Reactant for synthesis of phthalazinone derivatives as topically active phosphodiesterase 4 inhibitors
Reactant for:
- Quinoline selenium compounds
- Peripheral seratonin 5-HT3 receptor ligands
Reactant for synthesis of phthalazinone derivatives as topically active phosphodiesterase 4 inhibitors
Reactant for:
- Mannich reactions
- Asymmetric hydrogenation of quinolines
- Chemoselective reductive amination of carbonyl compounds
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Qingtong Wang et al.
Scientific reports, 7, 45364-45364 (2017-03-30)
T cell infiltration to synovial tissue is an early pathogenic mechanism of rheumatoid arthritis (RA). In the present work, we reveal that G protein coupled receptor kinase 2 (GRK2) is abundantly expressed in T cells of collagen-induced arthritis (CIA). A
Željko Pavković et al.
Progress in neuro-psychopharmacology & biological psychiatry, 96, 109733-109733 (2019-08-17)
Adolescent neurodevelopment confer vulnerability to the actions of treatments that produce adaptations in neurocircuitry underlying motivation, impulsivity and reward. Considering wide usage of a sedative-hypnotic agent propofol in clinical practice, we examined whether propofol is a challenging treatment for peripubertal
Reza Shamsimeymandi et al.
Archiv der Pharmazie, 352(7), e1800352-e1800352 (2019-05-29)
A series of novel chroman-4-one derivatives were designed and synthesized successfully with good to excellent yield (3a-l). In addition, the obtained products were evaluated for their cholinesterase (ChE) inhibitory activities. The results show that among the various synthesized compounds, analogs
Xiao-Xi Huang et al.
Nucleic acids research, 42(13), 8719-8731 (2014-06-19)
Ligands that can interact specifically with telomeric multimeric G-quadruplexes could be developed as promising anticancer drugs with few side effects related to other G-quadruplex-forming regions. In this paper, a new cationic porphyrin derivative, m-TMPipEOPP, was synthesized and characterized. Its multimeric
Global Trade Item Number
| SKU | GTIN |
|---|---|
| P46105-5G | |
| P46105-100G | 4061838355485 |
| P46105-500G |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service