P28263
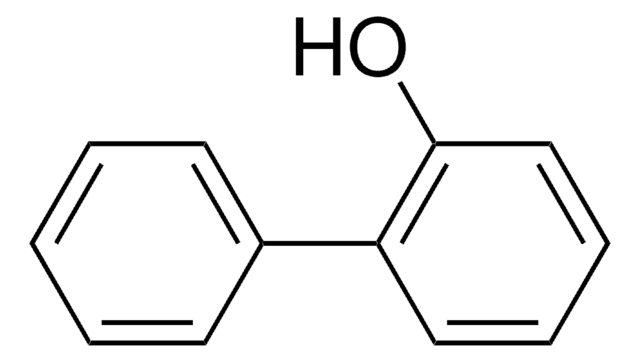
2-Phenylphenol
99%
Synonym(s):
2-Hydroxybiphenyl
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5C6H4OH
CAS Number:
Molecular Weight:
170.21
Beilstein:
606907
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
7 mmHg ( 140 °C)
Quality Level
Assay
99%
form
solid
bp
282 °C (lit.)
mp
57-59 °C (lit.)
fluorescence
λex 231 nm; λem 356 nm
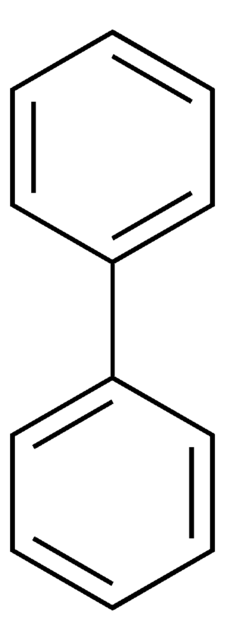
SMILES string
Oc1ccccc1-c2ccccc2
InChI
1S/C12H10O/c13-12-9-5-4-8-11(12)10-6-2-1-3-7-10/h1-9,13H
InChI key
LLEMOWNGBBNAJR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Phenylphenol, also known as o-phenylphenol, is commonly used as a chemical intermediate for the synthesis of pharmaceuticals, dyes, and fragrances.
Application
2-Phenylphenol can be used as a precursor in the synthesis of:
- Dibenzofurans via palladium-catalyzed, phenol-directed C-H activation/C-O cyclization or copper-catalyzed aerobic C-H activation, followed by cycloetherification.
- 6H-Dibenzo[b,d]pyran-6-ones via ruthenium-catalyzed carbonylative C-H cyclization.
- o-Phenylenes, a class of conjugated oligomers via an iterative sequence of Suzuki-Miyaura coupling reactions.
- o,o,p-Oligophenylenes via palladium-catalyzed C-H arylation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
255.2 °F - closed cup
Flash Point(C)
124 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Repetitive synthetic method for o, o, p-oligophenylenes using C?H arylation.
Manabe K and Kimura T
Organic Letters, 15(2), 374-377 (2013)
Ruthenium-Catalyzed Carbonylative C?H Cyclization of 2-Arylphenols: A Novel Synthetic Route to 6 H-Dibenzo [b, d] pyran-6-ones.
Inamoto K, et al.
Organic Letters, 15(15), 3962-3965 (2013)
Cu-catalyzed oxidative C (sp2)-H cycloetherification of o-arylphenols for the preparation of dibenzofurans.
Zhao J, et al.
Organic Letters, 14(4), 1078-1081 (2012)
Synthesis of dibenzofurans via Palladium-catalyzed phenol-directed C?H activation/C?O cyclization.
Xiao B, et al.
Journal of the American Chemical Society, 2011, 9250-9253 (2011)
Parent o-phenylene oligomers: synthesis, conformational behavior, and characterization.
Mathew S M and Hartley C S
Macromolecules, 44(21), 8425-8432 (2011)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service