P24055
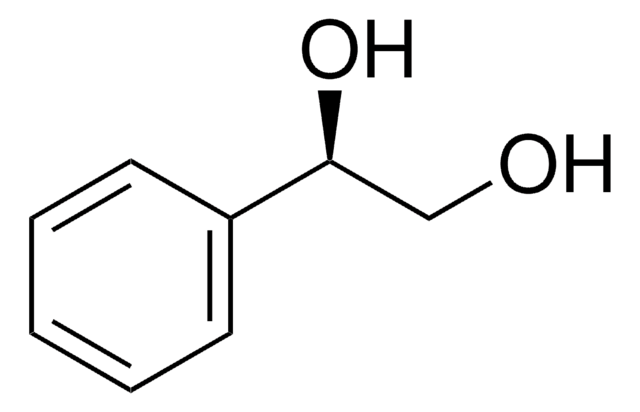
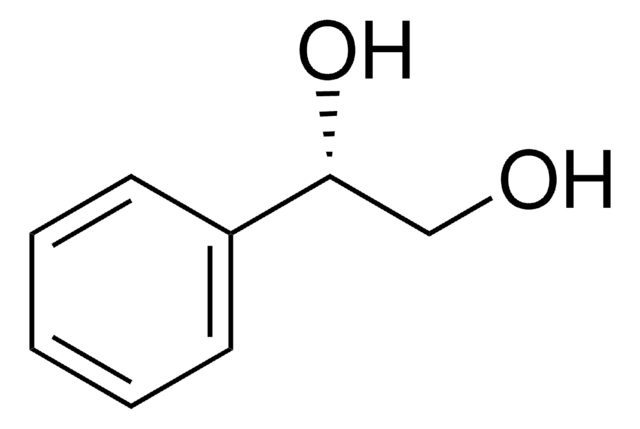
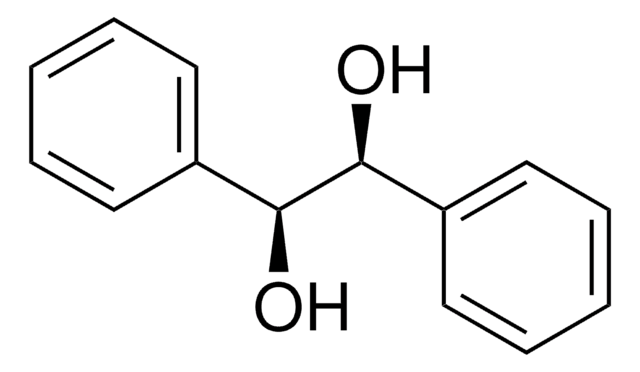
1-Phenyl-1,2-ethanediol
97%
Synonym(s):
(±)-1-Phenyl-1,2-ethanediol, (±)-Phenylethylene glycol
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6H5CH(OH)CH2OH
CAS Number:
Molecular Weight:
138.16
Beilstein:
1306723
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
bp
272-274 °C/760 mmHg (lit.)
mp
66-68 °C (lit.)
SMILES string
OCC(O)c1ccccc1
InChI
1S/C8H10O2/c9-6-8(10)7-4-2-1-3-5-7/h1-5,8-10H,6H2
InChI key
PWMWNFMRSKOCEY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ashwini C Mathpati et al.
Journal of biotechnology, 262, 1-10 (2017-09-30)
Kinetic resolution of rac-1,2-diols using the biocatalyst Burkholderia cepacia lipase (BCL) immobilized on a biodegradable binary blend support of hydroxypropyl methyl cellulose(HPMC)/polyvinyl alcohol (PVA) has been investigated. The immobilization technique improved enzyme activity significantly and it has excellent recyclability with
Cesar Mateo et al.
Analytical biochemistry, 314(1), 135-141 (2003-03-14)
In this paper we report the development of a novel and simple spectrophotometric assay which allows one to achieve the continuous, rapid, sensitive, and accurate determination of an epoxide hydrolase activity. This assay is based on the elaboration of a
Li Cao et al.
Biotechnology and bioengineering, 94(3), 522-529 (2006-02-25)
Soluble epoxide hydrolase (EH) from the potato Solanum tuberosum and an evolved EH of the bacterium Agrobacterium radiobacter AD1, EchA-I219F, were purified for the enantioconvergent hydrolysis of racemic styrene oxide into the single product (R)-1-phenyl-1,2-ethanediol, which is an important intermediate
Pablo Taboada et al.
Langmuir : the ACS journal of surfaces and colloids, 21(12), 5263-5271 (2005-06-01)
Three triblock copolymers of ethylene oxide and phenyl glycidyl ether, type E(m)G(n)E(m), where G = OCH2CH(CH2OC6H5) and E = OCH2CH2, were synthesized and characterized by gel-permeation chromatography, matrix-assisted laser desorption ionization time-of-flight mass spectrometry, and NMR spectroscopy. Their association properties
Qingsen Hu et al.
Bioresource technology, 101(21), 8461-8463 (2010-06-25)
In this study, a highly efficient process for Candida parapsilosis-catalyzed deracemization of racemic 1-phenyl-1,2-ethanediol (PED) was described, based on a resin-based in situ substrate feeding and product removal (ISSFPR) methodology. The resin H103 was selected and used to keep the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service