77292
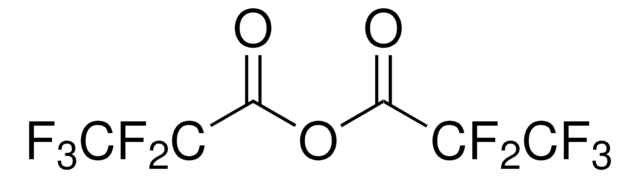
Pentafluoropropionic anhydride
purum, ≥97.0% (GC)
Synonym(s):
PFPA, Perfluoropropionic anhydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
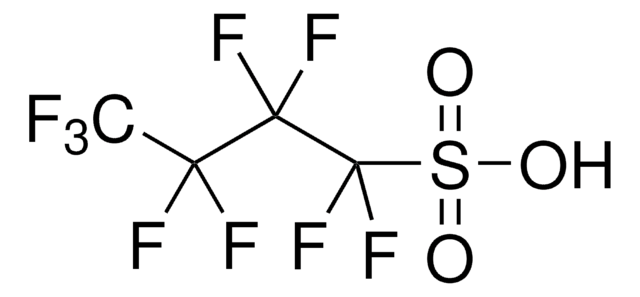
Linear Formula:
(CF3CF2CO)2O
CAS Number:
Molecular Weight:
310.05
Beilstein:
1806446
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥97.0% (GC)
form
liquid
bp
69-70 °C/735 mmHg (lit.)
density
1.571 g/mL at 25 °C (lit.)
functional group
anhydride
ester
fluoro
SMILES string
FC(F)(F)C(F)(F)C(=O)OC(=O)C(F)(F)C(F)(F)F
InChI
1S/C6F10O3/c7-3(8,5(11,12)13)1(17)19-2(18)4(9,10)6(14,15)16
InChI key
XETRHNFRKCNWAJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
PFPA acylates amines, amino acids and other compounds. PFPA derivatives are highly volatile; used in GC separation
Pentafluoropropionic anhydride can be used to synthesize lithium 4,5-dicyano-2-(pentafluoroethyl)imidazole, a novel imidazole-derived salt with potential application as an electrolyte in lithium-ion batteries. It can also be used to prepare 1.3- bis(pentafluoropropionyl)azulene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Supplementary Hazards
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemical Derivatization in Gas Chromatography.
J. Drozd et al.
J. Chromatogr. Library, 19 (1981)
New type of imidazole based salts designed specifically for lithium ion batteries.
Niedzicki L, et al.
Electrochimica Acta, 55(4), 1450-1454 (2010)
Simple syntheses of 1, 3-bis (perfluoroacyl) azulenes and 1, 3-azulenedicarboxylic acid.
Mathias L J and Overberger C G
The Journal of Organic Chemistry, 45(9), 1701-1703 (1980)
D.R. Knapp
Handbook of Analytical Derivatization Reactions (1979)
J. Drozd
J. Chromatogr. Library, 19 (1981)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service