695904
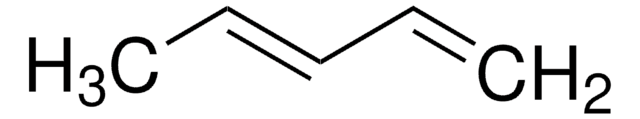
1,3-Butadiene solution
15 wt. % in hexane
Synonym(s):
Bivinyl, Vinylethylene, alpha,gamma-Butadiene
About This Item
Recommended Products
form
liquid
concentration
15 wt. % in hexane
refractive index
n20/D 1.376
density
0.682 g/mL at 25 °C
storage temp.
2-8°C
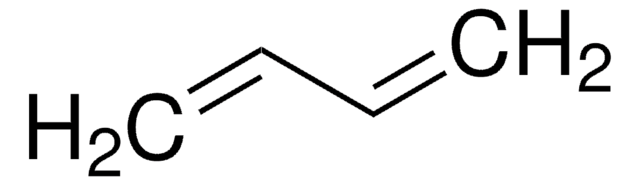
SMILES string
C=CC=C
InChI
1S/C4H6/c1-3-4-2/h3-4H,1-2H2
InChI key
KAKZBPTYRLMSJV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 2 - Muta. 1B - Repr. 2 - Skin Irrit. 2 - STOT RE 1 Inhalation - STOT SE 3
Target Organs
Central nervous system, Nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
-105.0 °F
Flash Point(C)
-76.11 °C
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
2-Butene; 2-Methylbutane; 1,3-Butadiene; Propyne
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service