59470
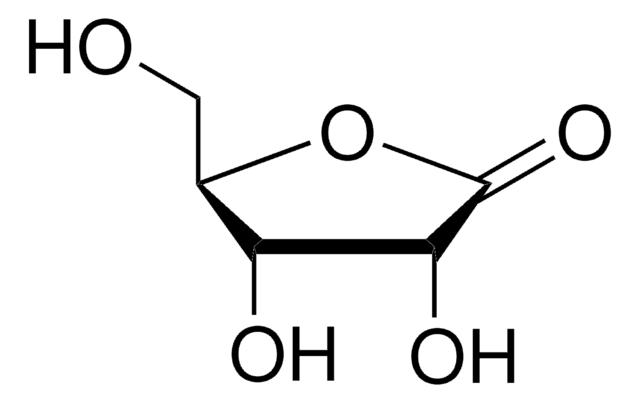
5,6-O-Isopropylidene-L-gulonic acid γ-lactone
≥99.0% (sum of enantiomers, TLC)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H14O6
CAS Number:
Molecular Weight:
218.20
Beilstein:
5267697
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99.0% (sum of enantiomers, TLC)
optical activity
[α]20/D +62±2°, c = 1% in H2O
mp
166-170 °C
SMILES string
CC1(C)OC[C@H](O1)[C@H]2OC(=O)[C@@H](O)[C@H]2O
InChI
1S/C9H14O6/c1-9(2)13-3-4(15-9)7-5(10)6(11)8(12)14-7/h4-7,10-11H,3H2,1-2H3/t4-,5+,6-,7+/m0/s1
InChI key
JNTPPVKRHGNFKM-BNHYGAARSA-N
Other Notes
Starting material for the synthesis of an array of compounds modified in the positions 2 and 3; synthesis of isopropylidene-L-glyceraldehyde
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The Journal of Organic Chemistry, 52, 1093-1093 (1987)
C. Hubschwerlen et al.
Organic Syntheses, 72, 1-1 (1995)
J.A. Vekemans et al.
Rec. Trav. Chim., 104, 266-266 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)