483869
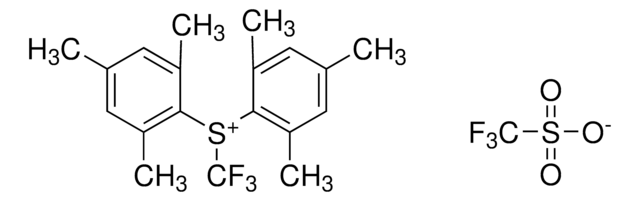
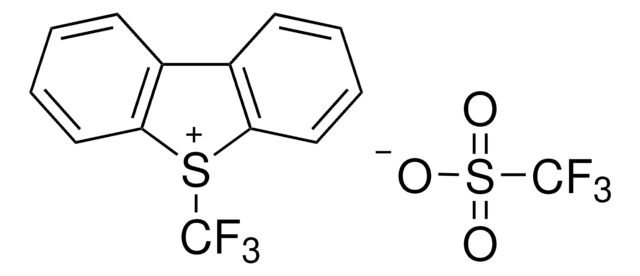
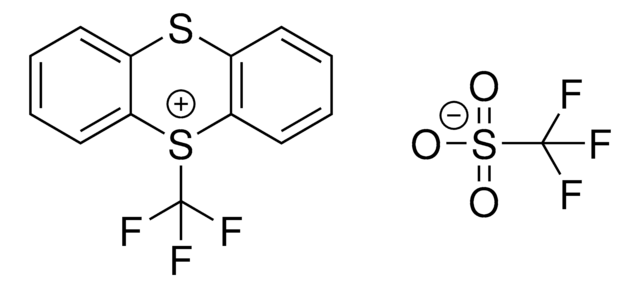
5-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate
97%
Synonym(s):
S-(Trifluoromethyl)dibenzothiophenium tetrafluoroborate, Umemoto reagent
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H8BF7S
CAS Number:
Molecular Weight:
340.07
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
162-164 °C (lit.)
functional group
fluoro
SMILES string
F[B-](F)(F)F.FC(F)(F)[S+]1c2ccccc2-c3ccccc13
InChI
1S/C13H8F3S.BF4/c14-13(15,16)17-11-7-3-1-5-9(11)10-6-2-4-8-12(10)17;2-1(3,4)5/h1-8H;/q+1;-1
InChI key
VTVISWLINKWMQZ-UHFFFAOYSA-N
Application
- Pd(II)-catalyzed trifluoromethylation
- Copper-catalyzed trifluoromethylation of aryl boronic acids using a Collidine as a trifluoromethylating reagent
- Pd-catalyzed electrophilic ortho-trifluoromethylation of arenes
Used in the stereoselective preparation of
- Trifluoromethyl-substituted alkenes via copper-catalyzed trifluoromethylation of terminal alkenes
- Trifluoromethyl-bearing quaternary carbon centers by Pd-catalyzed intramolecular decarboxylative allylation of α-trifluoromethyl β-keto esters
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jun Xu et al.
Chemical communications (Cambridge, England), 47(14), 4300-4302 (2011-03-08)
A copper-catalyzed process for trifluoromethylation of aryl, heteroaryl, and vinyl boronic acids has been developed. The reaction is conducted under mild conditions and shows tolerance to moisture and a variety of functional groups.
Construction of Trifluoromethyl-Bearing Quaternary Carbon Centers by Intramolecular Decarboxylative Allylation of α-Trifluoromethyl β-Keto Esters
Shibata, N.; et al.
Advanced Synthesis & Catalysis, 353, 2037-2041 (2011)
Xisheng Wang et al.
Journal of the American Chemical Society, 132(11), 3648-3649 (2010-02-27)
A Pd(II)-catalyzed C-H activation/trifluoromethylation of arenes with an electrophilic trifluoromethylation reagent using diverse heterocycle directing groups is reported. The presence of trifluoroacetic acid is crucial for this catalytic reaction.
Jun Xu et al.
Journal of the American Chemical Society, 133(39), 15300-15303 (2011-09-15)
An unprecedented type of reaction for Cu-catalyzed trifluoromethylation of terminal alkenes is reported. This reaction represents a rare instance of catalytic trifluoromethylation through C(sp(3))-H activation. It also provides a mechanistically unique example of Cu-catalyzed allylic C-H activation/functionalization. Both experimental and
Xing-Guo Zhang et al.
Journal of the American Chemical Society, 134(29), 11948-11951 (2012-07-12)
A Pd(II)-catalyzed trifluoromethylation of ortho C-H bonds with an array of N-arylbenzamides derived from benzoic acids is reported. N-Methylformamide has been identified as a crucial promoter of C-CF(3) bond formation from the Pd center. X-ray characterization of the C-H insertion
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service