476870
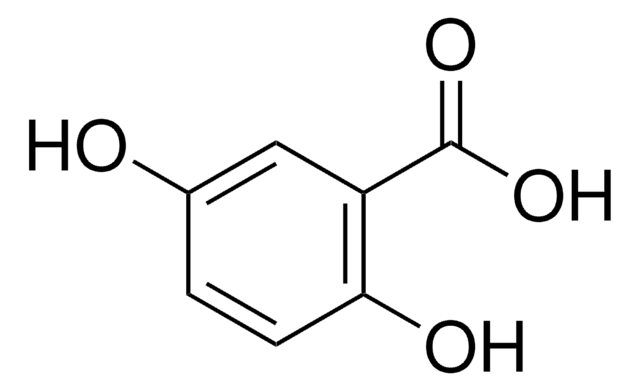
α-Cyano-4-hydroxycinnamic acid
99%
Synonym(s):
alpha-Cyano-4-hydroxycinnamic acid, α-CCA, α-CHCA, α-Cyano, 4-HCCA, ACCA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
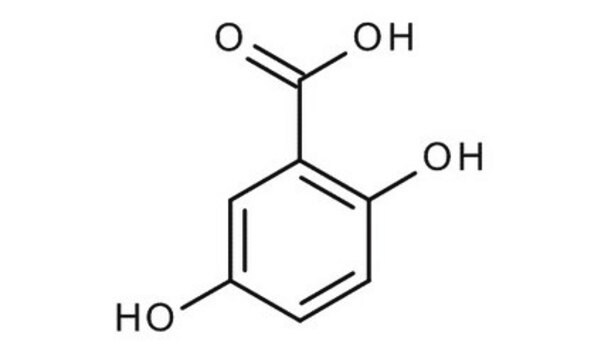
HOC6H4CH=C(CN)CO2H
CAS Number:
Molecular Weight:
189.17
Beilstein:
3271427
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
mp
245-250 °C (lit.)
functional group
carboxylic acid
nitrile
storage temp.
2-8°C
SMILES string
OC(=O)\C(=C\c1ccc(O)cc1)C#N
InChI
1S/C10H7NO3/c11-6-8(10(13)14)5-7-1-3-9(12)4-2-7/h1-5,12H,(H,13,14)/b8-5+
InChI key
AFVLVVWMAFSXCK-VMPITWQZSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
α-Cyano-4-hydroxycinnamic acid is the commonly used matrix for analyzing peptides by matrix-assisted laser desorption/ionization mass spectrometry. It inhibits the monophenolase activity and diphenolase activity of mushroom tyrosinase.
Application
α-Cyano-4-hydroxycinnamic acid (CHC) was used in encapsulation of CHC into NaY zeolite. It was used as matrix to investigate the activity of the paclitaxel derivatives using several well-established in vitro angiogenesis assays.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1B
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Inhibitory effects of α-cyano-4-hydroxycinnamic acid on the activity of mushroom tyrosinase.
Qiu L, et al.
Food Chemistry, 112(3), 609-613 (2009)
Encapsulation of α-cyano-4-hydroxycinnamic acid into a NaY zeolite.
Vilaca N, et al.
J. Mater. Sci., 46(23), 7511-7516 (2011)
Claudia Ryppa et al.
International journal of pharmaceutics, 368(1-2), 89-97 (2008-11-11)
The alpha(v)beta(3) integrin is overexpressed on proliferating endothelial cells such as those present in growing tumors as well as on tumor cells of various origins. Tumor-induced angiogenesis can be inhibited in vivo by antagonizing the alpha(v)beta(3) integrin with small peptides
Yusaku Hioki et al.
Journal of mass spectrometry : JMS, 48(11), 1217-1223 (2013-11-22)
We describe here an optimization study of the sample preparation conditions for sensitive detection of peptides by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS). Among many factors in the conditions, we varied the percent acetonitrile in the peptide solution, the percent
Cheng-Wei Chen et al.
International journal of nanomedicine, 6, 2567-2580 (2011-12-01)
Human retinal pigment epithelial cells are promising target sites for small interfering RNA (siRNA) that might be used for the prevention and/or treatment of choroidal neovascularization by inhibiting the expression of angiogenic factor; for example, by downregulating expression of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![trans-2-[3-(4-tert-Butylphenyl)-2-methyl-2-propenylidene]malononitrile matrix substance for MALDI-MS, ≥99.0% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/249/587/f8021369-f65a-413d-887d-3c8a4d2a248f/640/f8021369-f65a-413d-887d-3c8a4d2a248f.png)