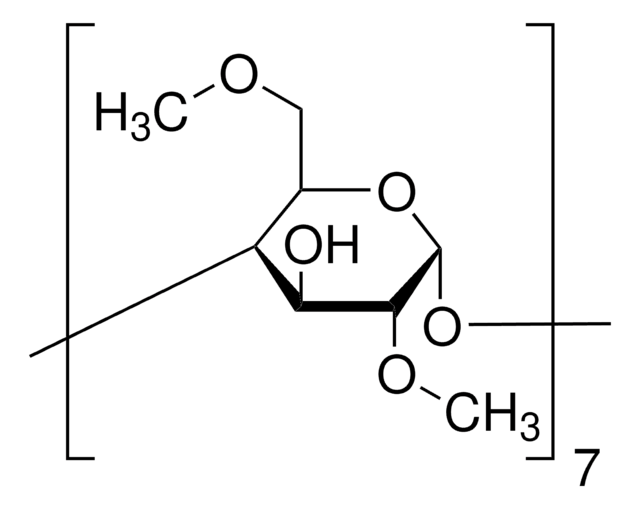
441562
trans-1-Acetyl-4-hydroxy-L-proline
≥98%, for peptide synthesis
Synonym(s):
N-Acetyl-L-hydroxyproline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H11NO4
CAS Number:
Molecular Weight:
173.17
Beilstein:
84043
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
trans-1-Acetyl-4-hydroxy-L-proline, ≥98%
Assay
≥98%
form
powder
optical activity
[α]20/D −119°, c = 4 in H2O
reaction suitability
reaction type: solution phase peptide synthesis
mp
132-133 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CC(=O)N1C[C@H](O)C[C@H]1C(O)=O
InChI
1S/C7H11NO4/c1-4(9)8-3-5(10)2-6(8)7(11)12/h5-6,10H,2-3H2,1H3,(H,11,12)/t5-,6+/m1/s1
InChI key
BAPRUDZDYCKSOQ-RITPCOANSA-N
Related Categories
Application
trans-1-Acetyl-4-hydroxy-L-proline can be used:
- In the stereospecific synthesis of 4-fluoroglutamic acid.
- To synthesize molecular targets for von Hippel-Lindau (VHL) E3 ubiquitin ligase.
- As a precursor to synthesize pseudopoly(amino acids) such as poly(trans-4-hydroxy-4-acyl-L-proline ester) and a biodegradable polymer, poly(lactic acid-glycolic acid-4-hydroxyproline).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Stereospecific syntheses of all four stereoisomers of 4-fluoroglutamic acid.
Hudlicky M.
Journal of Fluorine Chemistry, 60(2-3), 193-210 (1993)
Synthesis and characterization of a novel biodegradable polymer poly (lactic acid?glycolic acid?4?hydroxyproline).
Duan J, et al.
Journal of Applied Polymer Science, 103(6), 3585-3590 (2007)
Pseudopoly (amino acids): A Study of the Synthesis and Characterization of Poly (trans-4-hydroxy-N-acyl-L-proline esters).
Kwon H Y and Langer R
Macromolecules, 22(8), 3250-3255 (1989)
P Villani et al.
Presse medicale (Paris, France : 1983), 27(5), 211-214 (1998-10-13)
Assess the importance of the mid-term placebo effect of symptomatic slow acting drugs given for osteoarthritis. We analyzed six controlled trials available in the literature. Trial duration ranged from 2 to 6 months. The trials had been conducted to assess
[Leg ulcers caused by prolidase deficiency].
G Pasolini et al.
Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia, 123(10), 493-496 (1988-10-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service