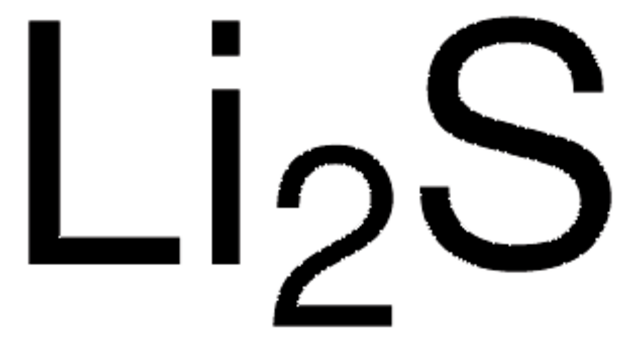439746
Lithium iodide
AnhydroBeads™, −10 mesh, 99.99% trace metals basis
Synonym(s):
Lithium monoiodide
About This Item
Recommended Products
product line
AnhydroBeads™
Quality Level
Assay
99.99% trace metals basis
form
beads
greener alternative product characteristics
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤150.0 ppm Trace Metal Analysis
particle size
−10 mesh
mp
446 °C (lit.)
density
3.49 g/mL at 25 °C (lit.)
greener alternative category
SMILES string
[Li+].[I-]
InChI
1S/HI.Li/h1H;/q;+1/p-1
InChI key
HSZCZNFXUDYRKD-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Application
- As a precursor to synthesize polymer-based electrolytes for dye-sensitized solar cell(DSSC) application via solution casting method.
- Li2S-P2S5-LiI crystalline inorganic-organic hybrid electrolytes with high ionic conductivity via liquid-phase synthesis for all solid-state batteries.
- As a redox mediator for Lithium–oxygen (Li–O2) batteries. It can facilitate redox reactions by shuttling charge carriers between electrodes, enabling efficient energy conversion.
Features and Benefits
- Excellent ionic conductivity at elevated temperature
- Good thermal stability
- Compatible with lithium-based battery materials.
Legal Information
accessory
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Research and development of solid-state lithium fast-ion conductors is crucial because they can be potentially used as solid electrolytes in all-solid-state batteries, which may solve the safety and energy-density related issues of conventional lithium-ion batteries that use liquid (farmable organic) electrolytes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service