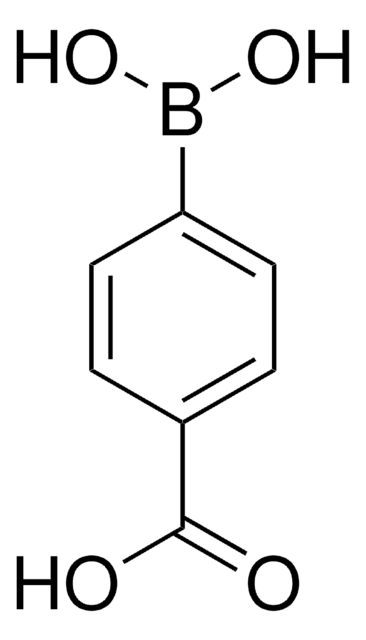
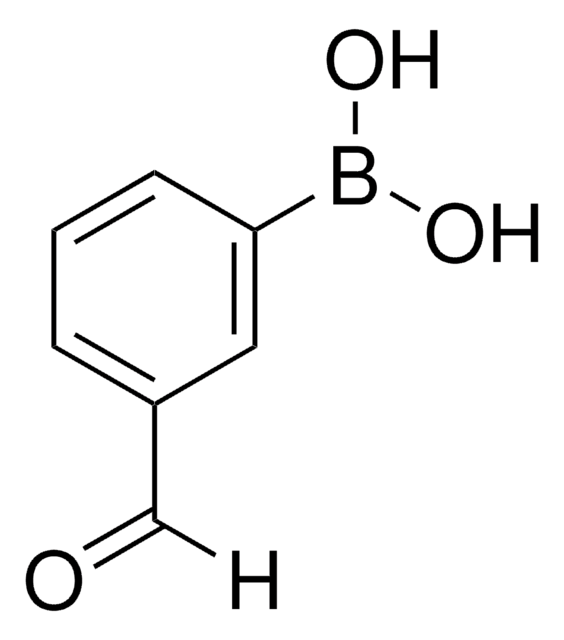
431966
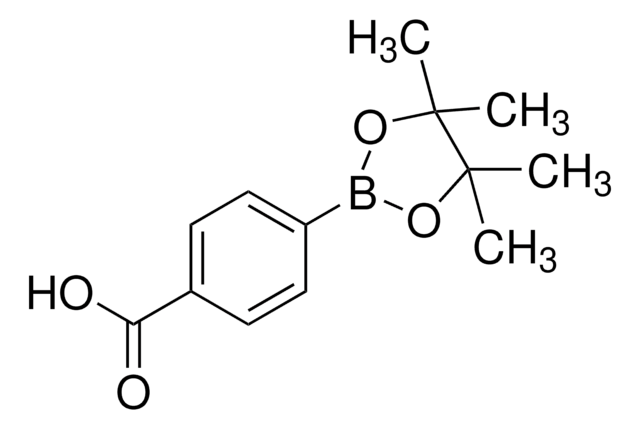
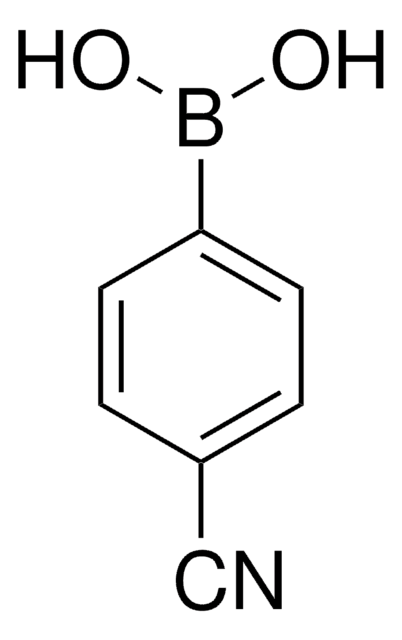
4-Formylphenylboronic acid
≥95.0%
Synonym(s):
4-(Dihydroxyboryl)benzaldehyde, 4-Boronobenzaldehyde, 4-Formylbenzeneboronic acid, p-Formylbenzeneboronic acid, p-Formylphenylboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HCOC6H4B(OH)2
CAS Number:
Molecular Weight:
149.94
Beilstein:
3030770
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
237-242 °C (lit.)
functional group
aldehyde
SMILES string
OB(O)c1ccc(C=O)cc1
InChI
1S/C7H7BO3/c9-5-6-1-3-7(4-2-6)8(10)11/h1-5,10-11H
InChI key
VXWBQOJISHAKKM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Formylphenylboronic acid is a substrate for Suzuki cross-coupling reactions and it can be used as a reagent for:
- Palladium-catalyzed Suzuki-Miyaura cross-coupling in water.
- Copper-mediated ligandless aerobic fluoroalkylation of arylboronic acids with fluoroalkyl iodides.
- Ligand-free copper-catalyzed coupling of nitro arenes with arylboronic acids.
- Triethylamine-catalyzed three-component Hantzsch condensations.
- Copper-catalyzed nitrations.
- Oxidative mono-cleavage of dialkenes catalyzed by Trametes hirsuta.
- Palladacycle-catalyzed cross-coupling of arylboronic acids with carboxylic anhydrides or acyl chlorides.
- Palladium-catalyzed aerobic oxidative cross-coupling reactions.
- The synthesis of sensitizers with dithiafulvenyl unit as electron donor for high-efficiency dye-sensitized solar cells.
- The synthesis of a novel protein synthesis inhibitor active against Gram-positive bacteria.
- The Suzuki aryl-aryl coupling of the upper rim of hexahomotrioxacalix[3]arene.
- A rhodium-catalyzed cyclization, converting 1,5-enynes to cyclopentenes and spiro-cyclopentenes.
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of Some New 1,4-Dihydropyridine Derivatives through a Facile One-pot Hantzsch Condensation Catalyzed by Triethylamine
Chin. J. Chem., 30, 733-737 (2012)
Kunpeng Guo et al.
Organic letters, 14(9), 2214-2217 (2012-04-14)
This work identifies the dithiafulvenyl unit as an excellent electron donor for constructing D-π-A-type metal-free organic sensitizers of dye-sensitized solar cells (DSCs). Synthesized and tested are three sensitizers all with this donor and a cyanoacrylic acid acceptor but differing in
Tetrahedron, 62, 10321-10321 (2006)
Nora R Eibergen et al.
Chembiochem : a European journal of chemical biology, 13(4), 574-583 (2012-03-01)
In an effort to identify novel antibacterial chemotypes, we performed a whole-cell screen for inhibitors of Staphylococcus aureus growth and pursued those compounds with previously uncharacterized antibacterial activity. This process resulted in the identification of a benzothiazolium salt, ABTZ-1, that
Qi Huang et al.
ACS applied materials & interfaces, 11(17), 15861-15868 (2019-03-28)
Conjugated microporous polymers (CMPs) with high surface areas, tunable building blocks, and fully conjugated structures have found important applications in optoelectronics. Here, we report a new series of CMPs with tunable band gaps by introducing thiazolo[5,4- d] thiazole as the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service