All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H20S
CAS Number:
Molecular Weight:
196.35
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.492 (lit.)
bp
106-107 °C/3 mmHg (lit.)
density
0.92 g/mL at 25 °C (lit.)
SMILES string
CCCCCCCCc1ccsc1
InChI
1S/C12H20S/c1-2-3-4-5-6-7-8-12-9-10-13-11-12/h9-11H,2-8H2,1H3
InChI key
WQYWXQCOYRZFAV-UHFFFAOYSA-N
General description
3-Octylthiophene is an alkyl thiophene derivative. It has been synthesized by the reaction of 3-bromothiophene with octylmagnesium bromide.
Application
3-Octylthiophene may be used as a starting material in the synthesis of the following:
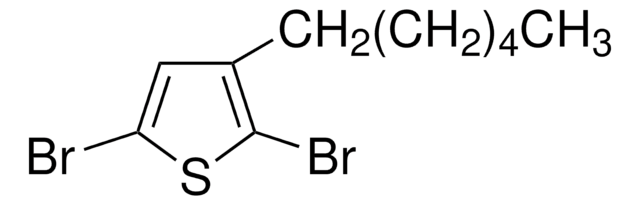
- 2,5-dibromo-3-octylthiophene
- poly(3-butylthiophene)-b-poly(3-octylthiophene), a diblock copoly(3-alkylthiophene)
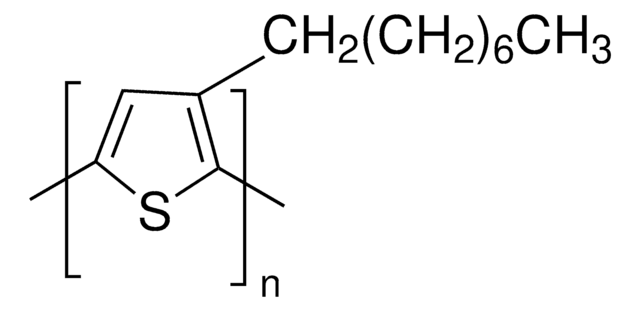
- regioregular poly(3-octylthiophene) (POT)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Crystalline diblock conjugated copolymers: Synthesis, self-assembly, and microphase separation of poly (3-butylthiophene)-b-poly (3-octylthiophene).
Wu PT, et al.
Macromolecules, 42(7), 2317-2320 (2009)
Soluble Phenanthrenyl-Imidazole-Presenting Regioregular Poly (3-octylthiophene) Copolymers Having Tunable Bandgaps for Solar Cell Applications.
Chang YT, et al.
Advances in Functional Materials, 17(16), 3326-3331 (2007)
New convenient synthesis of highly regioregular poly (3-octylthiophene) based on the Suzuki coupling reaction.
Guillerez S and Bidan G.
Synthetic Metals, 93(2), 123-126 (1998)
Nannan Jian et al.
Physical chemistry chemical physics : PCCP, 21(13), 7174-7182 (2019-03-20)
Conjugated fluorophores have been extensively used for fluorescence sensing of various substances in the field of life processes and environmental science, due to their noninvasiveness, sensitivity, simplicity and rapidity. Most existing conjugated fluorophores exhibit excellent light-emitting performance in dilute solutions
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service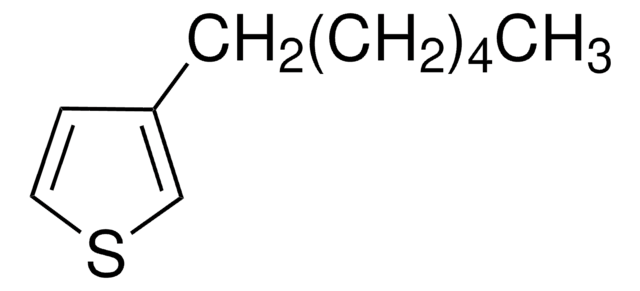

![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate Selectophore™](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)

