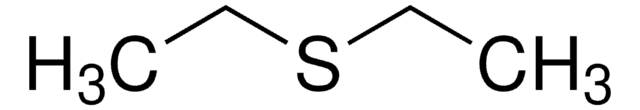416452
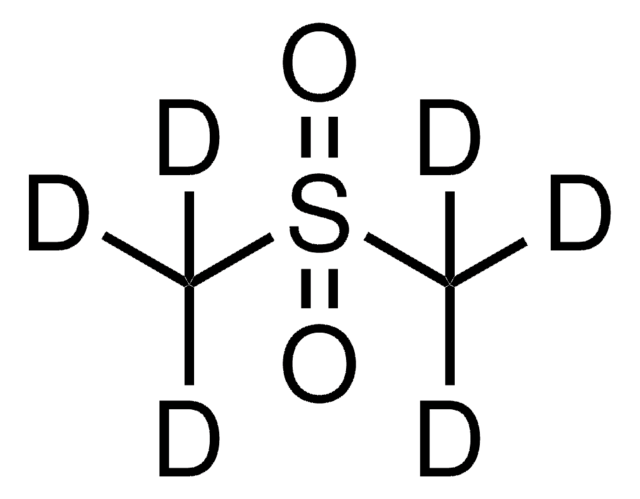
Dimethyl sulfide-d6
99 atom % D
Synonym(s):
(Methyl sulfide)-d6, Hexadeuterodimethyl sulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CD3SCD3
CAS Number:
Molecular Weight:
68.17
Beilstein:
2036892
EC Number:
MDL number:
UNSPSC Code:
12142201
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
isotopic purity
99 atom % D
Quality Level
Assay
99% (CP)
form
liquid
technique(s)
NMR: suitable
refractive index
n20/D 1.431 (lit.)
bp
36.5 °C (lit.)
density
0.928 g/mL at 25 °C (lit.)
mass shift
M+6
SMILES string
[2H]C([2H])([2H])SC([2H])([2H])[2H]
InChI
1S/C2H6S/c1-3-2/h1-2H3/i1D3,2D3
InChI key
QMMFVYPAHWMCMS-WFGJKAKNSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Dimethyl sulfide-d6 [(CD3)2S] is a deuterated NMR solvent useful in NMR-based research and analyses. It can form 1:1 complex with halothane by the formation of C–H…S hydrogen bonds. During the reaction of (CD3)2S with singlet oxygen in aprotic solvents, the H-D exchange in the methyl group was observed. The electronic spectra of (CD3)2S has been measured between 1250 and 2500Å.
Recommended products
Check out ChemisTwin®, our brand new online portal for identity confirmation and quantification of NMR spectra. Learn more or reach out to us for a free trial.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-34.6 °F - closed cup
Flash Point(C)
-37 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Vibrational spectra of (CH3)2S, CH3SCD3, and (CD3)2S.
Ypenburg JW and Gerding H.
Rec. Trav. Chim., 90(8), 885-895 (1971)
Mechanism of sulfone formation in the reaction of sulfides and singlet oxygen: intermediacy of S-hydroperoxysulfonium ylide.
Ishiguro K, et al.
Journal of the American Chemical Society, 118(31), 7265-7271 (1996)
Intramolecular potential function and methyl-methyl coupling of crystalline dimethyl sulfide.
Levin IW, et al.
Spectrochimica Acta Part A: Molecular Spectroscopy, 31(1), 41-55 (1975)
Assignments of Rydberg and valence transitions in the electronic absorption spectrum of dimethyl sulfide.
McDiarmid R.
J. Chem. Phys., 61(1), 274-281 (1974)
C-H?X (X= S, P) hydrogen bonding: The complexes of halothane with dimethyl sulfide and trimethylphosphine.
Michielsen B, et al.
Journal of Molecular Structure, 1023, 90-95 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service