408166
1-(2-Pyridyl)piperazine
≥99%
Synonym(s):
2-Piperazinopyridine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H13N3
CAS Number:
Molecular Weight:
163.22
Beilstein:
140423
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
liquid
refractive index
n20/D 1.595 (lit.)
bp
120-122 °C/2 mmHg (lit.)
density
1.072 g/mL at 25 °C (lit.)
SMILES string
C1CN(CCN1)c2ccccn2
InChI
1S/C9H13N3/c1-2-4-11-9(3-1)12-7-5-10-6-8-12/h1-4,10H,5-8H2
InChI key
GZRKXKUVVPSREJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-(2-Pyridyl)piperazine is a piperazine derivative.
Application
1-(2-Pyridyl)piperazine may be employed as reagent for the determination of both aliphatic and aromatic isocyanates in air by reversed-phase HPLC., It may be employed as reagent for the fluorometric determination of airborne diisocyantes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of trace atmospheric isocyanate concentrations by reversed-phase high-performance liquid chromatography using 1-(2-pyridyl) piperazine reagent.
Goldberg PA, et al.
Journal of Chromatography A, 212(1), 93-104 (1981)
Absorption and fluorescence of 1-(2-pyridyl)-piperazine and four diisocyanate derivatives in solution.
Salthammer T, et al.
Journal of Photochemistry and Photobiology A: Chemistry, 107(1), 159-164 (1997)
Determination of atmospheric isocyanate concentrations by high-performance thin-layer chromatography using 1-(2-pyridyl) piperazine reagent.
Ellwood PA, et al.
Analyst, 106(1258), 85-93 (1981)
Robert Lavieri et al.
Bioorganic & medicinal chemistry letters, 19(8), 2240-2243 (2009-03-21)
This Letter describes the synthesis and structure-activity relationships (SAR) of isoform-selective PLD inhibitors. By virtue of the installation of a 1,3,8-triazaspiro[4,5]decan-4-one privileged structure, PLD inhibitors with nanomolar potency and an unprecedented 40-fold selectivity for PLD2 over PLD1 were developed. Interestingly
P Giral et al.
European journal of pharmacology, 134(1), 113-116 (1987-01-28)
We investigated in mice the effects of one of the principal metabolites of buspirone and gepirone, 1-(2-pyridinyl)-piperazine (1-PmP), on hypothermia and reduced locomotion induced by clonidine (0.25 and 0.06 mg/kg, respectively), tests related to brain alpha-adrenergic function. Both effects were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service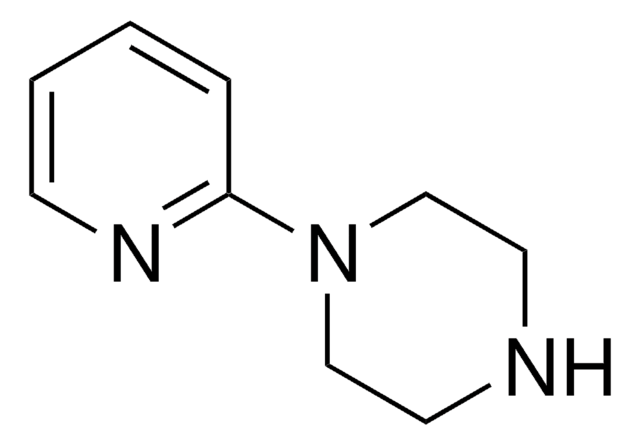

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)