All Photos(1)
About This Item
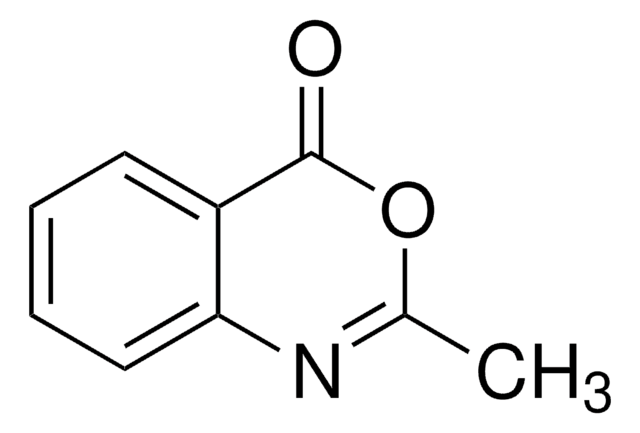
Empirical Formula (Hill Notation):
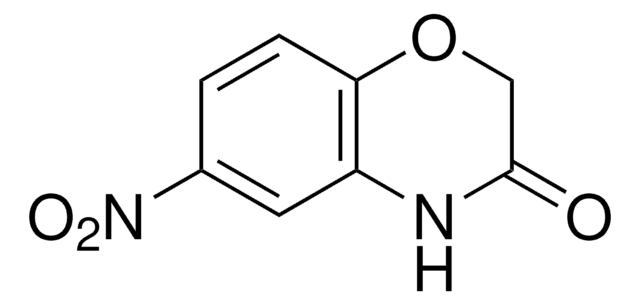
C8H7NO2
CAS Number:
Molecular Weight:
149.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
173-175 °C (lit.)
solubility
methanol: soluble 25 mg/mL, clear, colorless
SMILES string
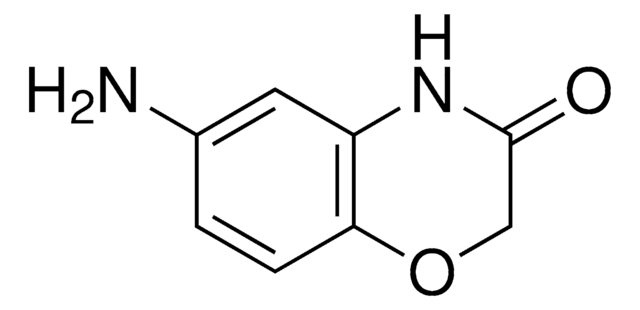
O=C1COc2ccccc2N1
InChI
1S/C8H7NO2/c10-8-5-11-7-4-2-1-3-6(7)9-8/h1-4H,5H2,(H,9,10)
InChI key
QRCGFTXRXYMJOS-UHFFFAOYSA-N
Related Categories
General description
2H-1,4-Benzoxazin-3(4H)-one, a benzoxazine derivative, is a heterocyclic building block for various natural and synthetic organic compounds. It has been reported as an intermediate during the biogenesis of cyclic hydoxamic acids in maize. Its standard molar enthalpy of formation and tautomerization energy of its tautomers has been evaluated by calorimetric and computational methods. It has been synthesized by reacting o-aminophenol with chloroacetyl chloride in the presence of butanone and aqueous NaHCO3.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
2H-1,4-benzoxazin-3(4H)-one, an intermediate in the biosynthesis of cyclic hydroxamic acids in maize.
Kumar P, et al.
Phytochemistry, 36(4), 893-898 (1994)
A general and convenient synthesis of 2H-1,4-benzoxazin-3(4H)-ones.
Shridhar DR, et al.
Organic Prep. and Proc. Int., 14(3), 195-197 (1982)
Jiu Hong Wu et al.
Bioorganic & medicinal chemistry letters, 13(13), 2223-2225 (2003-06-12)
A new inhibitor of in vitro tumor cell replication, cappamensin A (1) (2H-1,4-benzoxazin-3(4H)-one, 6-methoxy-2-methyl-4-carbaldehyde), was isolated from the roots of Capparis sikkimensis subsp. formosana using bioactivity-guided fractionation. The structure of 1 was established by spectroscopic methods, including 2D NMR analyses.
Calorimetric and computational study of 2H-1,4-benzoxazin-3(4H)-one and of related species.
Matos MAR, et al.
Molecular Physics, 104(12), 1833-1841 (2006)
Recent advances in the synthesis of 2H-1, 4-benzoxazin-3-(4H)-ones and 3, 4-dihydro-2 H -1, 4-benzoxazines.
Ilas J, et al.
Tetrahedron, 61(31), 7325-7348 (2005)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service