360899
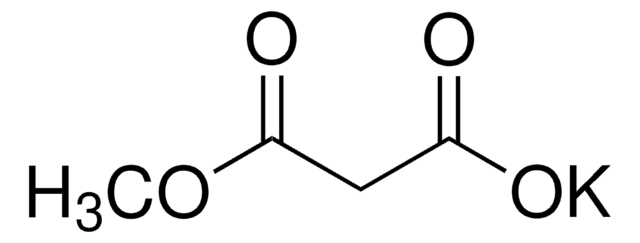
Ethyl potassium malonate
98%
Synonym(s):
Monoethyl malonate potassium salt, Potassium monoethyl malonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C2H5OCOCH2CO2K
CAS Number:
Molecular Weight:
170.20
Beilstein:
3721682
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
194 °C (dec.) (lit.)
functional group
ester
SMILES string
[K+].CCOC(=O)CC([O-])=O
InChI
1S/C5H8O4.K/c1-2-9-5(8)3-4(6)7;/h2-3H2,1H3,(H,6,7);/q;+1/p-1
InChI key
WVUCPRGADMCTBN-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl potassium malonate (potassium ethyl malonate) reacts with aryl nitriles in the presence of zinc chloride and a catalytic amount of Hünig′s base to yield β-amino acrylates. Ethyl potassium malonate is formed as an intermediate during the synthesis of ethyl tert-butyl malonate.
Application
Ethyl potassium malonate (potassium ethyl malonate) may be used to generate (trimethylsilyl)ethyl malonate in situ, which can be acylated to prepare a variety of β-ketoesters or alkylidene malonates.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A process for the synthesis of ?-ketoesters using in-situ generated (trimethylsilyl) malonates
Wang X, et al.
Tetrahedron Letters, 35(50), 9323-9326 (1994)
Jae Hoon Lee et al.
The Journal of organic chemistry, 72(26), 10261-10263 (2007-12-01)
Reaction of aryl nitriles with potassium ethyl malonate in the presence of zinc chloride and a catalytic amount of Hünig's base provided beta-amino acrylates in moderate to good yield. Compared to the classical Blaise reaction, this reaction is safer (endothermic)
Ethyl tert-Butyl Malonate.
Strube RE.
Organometallic Syntheses, 34-34 (1963)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service