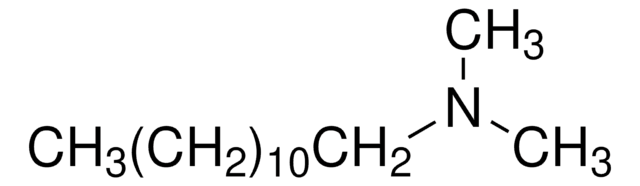359327
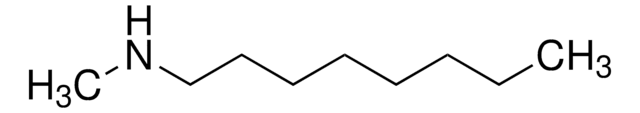
N-Hexylmethylamine
96%
Synonym(s):
N-Methylhexylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3NH(CH2)5CH3
CAS Number:
Molecular Weight:
115.22
Beilstein:
1731685
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.416 (lit.)
bp
140-142 °C (lit.)
density
0.76 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CCCCCCNC
InChI
1S/C7H17N/c1-3-4-5-6-7-8-2/h8H,3-7H2,1-2H3
InChI key
XJINZNWPEQMMBV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
N-Hexylmethylamine is an acyclic secondary amine. Ruthenium-catalyzed reaction of N-hexylmethylamine with styrene has been reported. Transition metal-catalyzed intermolecular hydroamination of N-hexylmethylamine with 2-vinylnaphthalene has been reported.
Application
N-Hexylmethylamine may be used in the synthesis of dialkyldithiocarbamato cadmium complexes Cd[S2CNRR′]2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
87.8 °F - closed cup
Flash Point(C)
31 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Masaru Utsunomiya et al.
Journal of the American Chemical Society, 126(9), 2702-2703 (2004-03-05)
A ruthenium-catalyzed intermolecular, anti-Markovnikov hydroamination of vinylarenes with secondary aliphatic and benzylic amines is reported. The combination of Ru(cod)(2-methylallyl)2, 1,5-bis(diphenylphosphino)pentane, and triflic acid was the most effective catalyst of those tested. Control reactions conducted without ligand or acid did not
The synthesis of SiO2@ CdS nanocomposites using single-molecule precursors.
Monteiro OC, et al.
Chemistry of Materials, 14(7), 2900-2904 (2002)
Masaru Utsunomiya et al.
Journal of the American Chemical Society, 125(47), 14286-14287 (2003-11-20)
A transition metal-catalyzed intermolecular hydroamination of vinylarenes with alkylamines is reported. The combination of Pd(O2CCF3)4, DPPF, and TfOH was the most effective catalyst of those tested. Control experiments without palladium, acid, or ligand all occurred in low yield. The reaction
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 359327-10ML | 4061831813227 |
| 359327-2ML |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service