All Photos(1)
About This Item
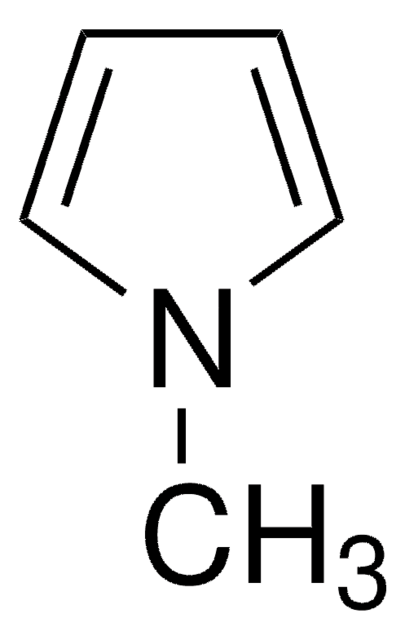
Empirical Formula (Hill Notation):
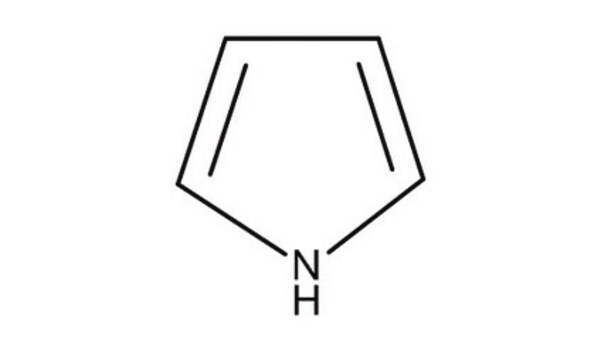
C6H9N
CAS Number:
Molecular Weight:
95.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
liquid
refractive index
n20/D 1.497 (lit.)
bp
164-165 °C/754 mmHg (lit.)
density
0.929 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CCc1ccc[nH]1
InChI
1S/C6H9N/c1-2-6-4-3-5-7-6/h3-5,7H,2H2,1H3
InChI key
XRPDDDRNQJNHLQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Ethylpyrrole is a monosubstituted alkyl pyrrole. Stochastic, Rothemund-type condensation of pyrrole, 2-ethylpyrrole and arylaldehyde yields 3-ethyl-substituted inverted porphyrin, which readily converts to 3-(1′-hydroxyethyl)- and 3-acetyl-inverted porphyrins. 2-Ethylpyrrole is a conjugated five-membered heterocyclic flavor compound and its photooxidation reactivity was investigated.
Application
2-Ethylpyrrole may be used in the preparation of 1-(3-methoxyphenyl)-2-ethylpyrrole.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
143.6 °F - closed cup
Flash Point(C)
62 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Reactivity on photooxidation of selected five-membered heterocyclic flavor compounds.
Chen C-W and Ho C-T.
Journal of Agricultural and Food Chemistry, 44(8), 2078-2080 (1996)
Application of 2-ethylpyrrole for a direct synthesis of 3-substituted inverted porphyrins.
Schmidt I and Chmielewski PJ.
Tetrahedron Letters, 42(36), 6389-6392 (2001)
Jon C Antilla et al.
The Journal of organic chemistry, 69(17), 5578-5587 (2004-08-17)
This paper details the copper-catalyzed N-arylation of pi-excessive nitrogen heterocycles. The coupling of either aryl iodides or aryl bromides with common nitrogen heterocycles (pyrroles, pyrazoles, indazoles, imidazoles, and triazoles) was successfully performed in good yield with catalysts derived from diamine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service