328774
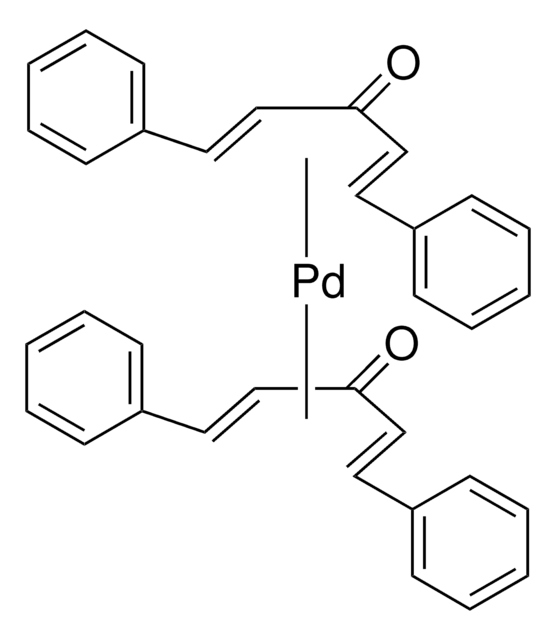
Tris(dibenzylideneacetone)dipalladium(0)
97%
Synonym(s):
Pd2dba3, Pd2(dba)3
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
core: palladium
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
mp
152-155 °C (lit.)
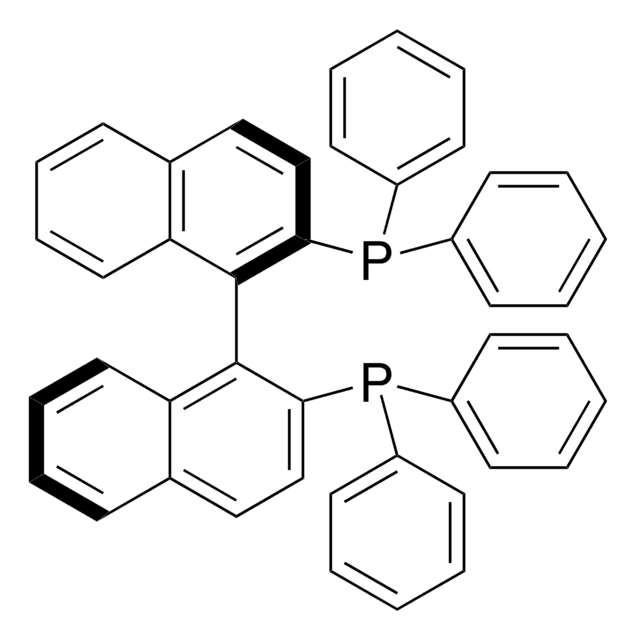
SMILES string
[Pd].[Pd].O=C(\C=C\c1ccccc1)/C=C/c2ccccc2.O=C(\C=C\c3ccccc3)/C=C/c4ccccc4.O=C(\C=C\c5ccccc5)/C=C/c6ccccc6
InChI
1S/3C17H14O.2Pd/c3*18-17(13-11-15-7-3-1-4-8-15)14-12-16-9-5-2-6-10-16;;/h3*1-14H;;/b3*13-11+,14-12+;;
InChI key
CYPYTURSJDMMMP-WVCUSYJESA-N
Looking for similar products? Visit Product Comparison Guide
General description
For small scale and high throughput uses, product is also available as ChemBeads (919772)
Application
- Application Guide for Palladium Catalyzed Cross-Coupling Reactions
- Synthesis of azepanes
- Synthesis of nanosized palladium phosphides upon interaction with white phosphorous
- Preparation of palladium triphenylphosphine carbonyl cluster complexes
- Precursor for synthesis of functionalized multiwalled carbon nanotube-palladium complexes used as catalysts for Heck coupling reactions
- Selective carbon-sulfur bond formation via addition of S-S and S-H bonds to alkynes
Reactant involved in:
- Catalyst for:
- Suzuki cross-coupling reactions
- PCN- and PCS-pincer palladium complex catalyzed tandem allylation
- Catalyst for Suzuki coupling of aryl chlorides (eq. 1)
- Catalyst for Heck coupling of aryl chlorides (eq. 2)
- Catalyst for arylation of ketones (eq. 3)
- Catalyst for Buchwald-Hartwig amination of aryl halides (eq. 4)
- Catalyst for fluorination of allylic chlorides (eq. 5)
- Catalyst for β-arylation of carboxylic esters (eq. 6)
- Catalyst for carbonylation of 1,1-dichloro-1-alkenes (eq. 7)
- Catalyst for conversion of aryl and vinyl triflates to aryl and vinyl halides (eq. 8)
- Pd source for enantioselective Tsuji Allylations

Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
JosiPhos CyPF-tBu and palladium give catalyst for alkoxylation of activated heteroaryl halides with primary, secondary, and tertiary alcohols
The Heck reaction is the palladium catalyzed cross-coupling reaction between alkenes and aryl or vinyl halides (or triflates) to afford substituted alkenes.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

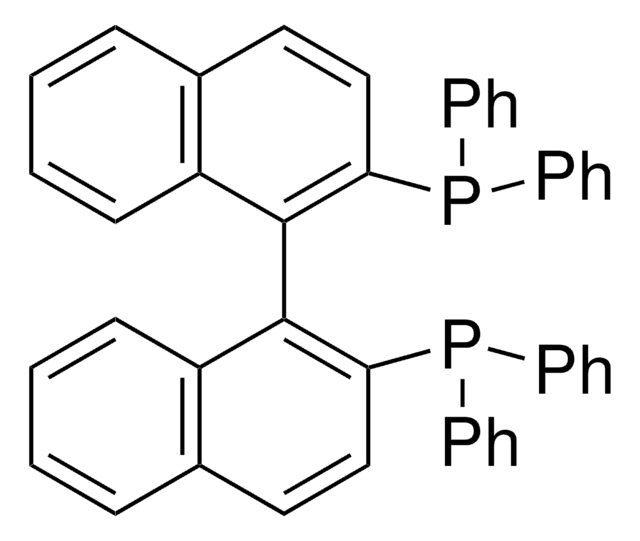
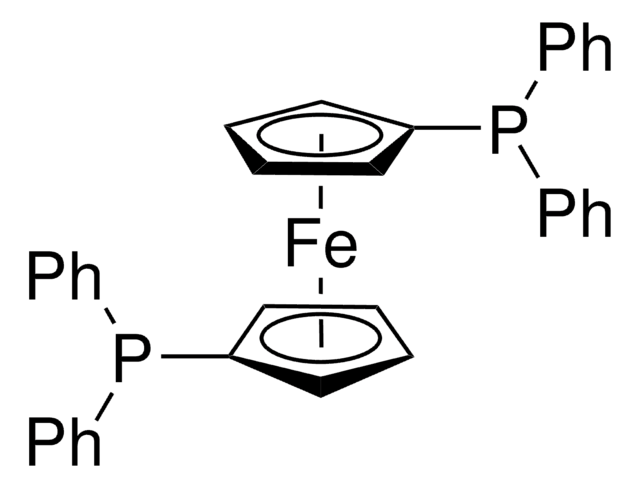
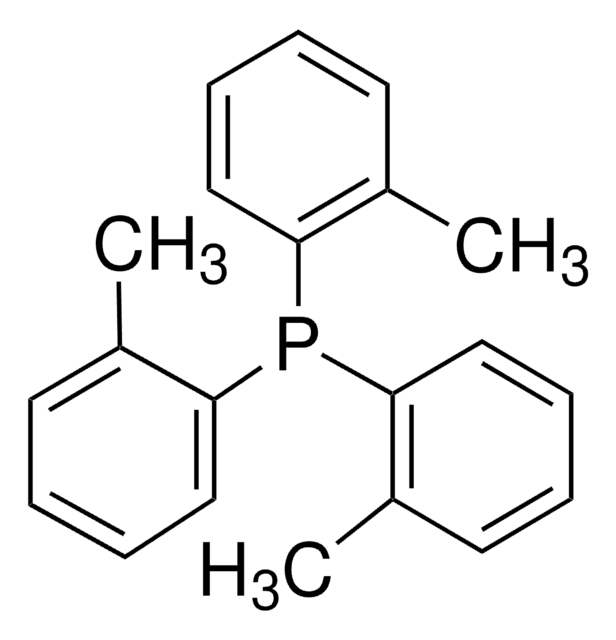
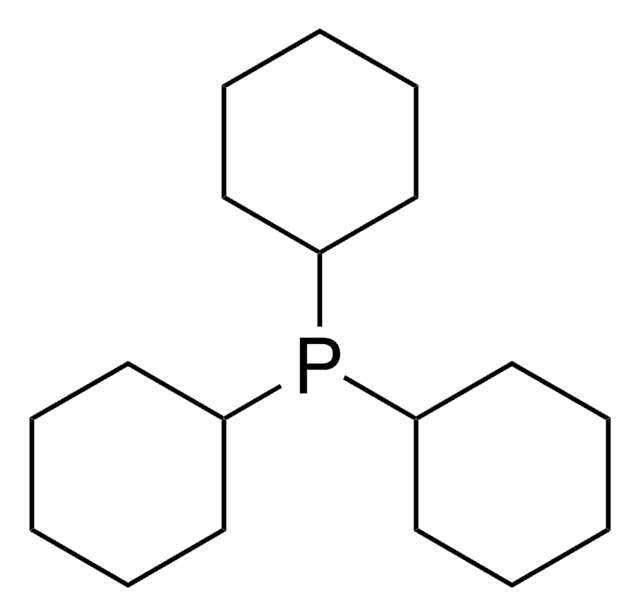
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)