303461
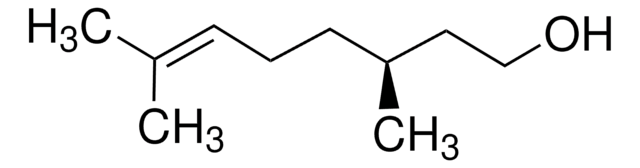
(R)-(+)-β-Citronellol
95%
Synonym(s):
(+)-β-Citronellol, (R)-3,7-Dimethyl-6-octen-1-ol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
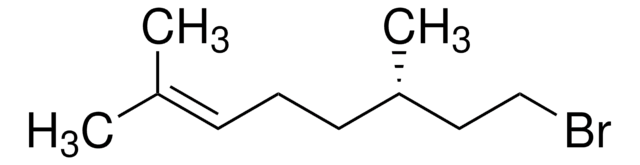
(CH3)2C=CHCH2CH2CH(CH3)CH2CH2OH
CAS Number:
Molecular Weight:
156.27
Beilstein:
1721506
EC Number:
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor pressure
~0.02 mmHg ( 25 °C)
Quality Level
Assay
95%
form
liquid
optical activity
[α]19/D +5.3°, neat
refractive index
n20/D 1.456 (lit.)
bp
112-113 °C/12 mmHg (lit.)
density
0.857 g/mL at 25 °C (lit.)
SMILES string
C[C@@H](CCO)CC\C=C(\C)C
InChI
1S/C10H20O/c1-9(2)5-4-6-10(3)7-8-11/h5,10-11H,4,6-8H2,1-3H3/t10-/m1/s1
InChI key
QMVPMAAFGQKVCJ-SNVBAGLBSA-N
Related Categories
Application
(R)-(+)-β-Citronellol can be used to prepare:
It can also be used as a starting material for the synthesis of (+)-integerrinecic acid lactone.
- (R)-(+)-β-citronellyl acetate by treating with vinyl acetate via trans esterification reaction using immobilized Rhizomucor miehei lipase as biocatalyst.
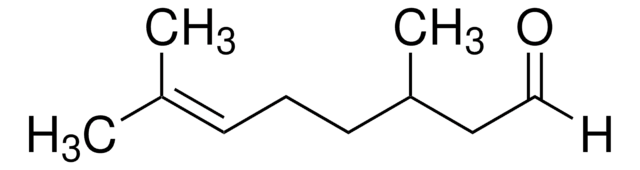
- (R)-(+)-citronellal and (R)-(+)-citronellic acid by the oxidation reaction.
- Citronellol aziridine by treating with 2,2,2-trichloroethoxysulfonamide by intermolecular olefin aziridination.
It can also be used as a starting material for the synthesis of (+)-integerrinecic acid lactone.
(R)-(+)-β-Citronellol can undergo:
- Oxidation to form (R)-(+)-citronellal, an antifungal agent and (R)-(+)-citronellic acid.
- Intermolecular olefin aziridination with 2,2,2-trichloroethoxysulfonamide to form citronellol aziridine for ring-opening reactions.
- Series of reactions to form α2δ-ligands for treating generalized anxiety disorder and insomnia.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
212.0 °F - closed cup
Flash Point(C)
100 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phenylglycine methyl ester, a useful tool for absolute configuration determination of various chiral carboxylic acids.
Yabuuchi T and Kusumi T
The Journal of Organic Chemistry, 65(2), 397-404 (2000)
Rh-catalyzed alkene oxidation: a highly efficient and selective process for preparing N-alkoxysulfonyl aziridines.
Guthikonda K, et al.
Tetrahedron, 62(49), 11331-11342 (2006)
A novel synthesis of (+)-integerrinecic acid lactone from R-(+) β-citronellol
White JD and Jayasinghe LR
Tetrahedron Letters, 29(18), 2139-2142 (1988)
Abir B Majumder et al.
Chemistry Central journal, 1, 10-10 (2007-09-21)
Use of enzymes in low water media is now widely used for synthesis and kinetic resolution of organic compounds. The frequently used enzyme form is the freeze-dried powders. It has been shown earlier that removal of water molecules from enzyme
A very mild and chemoselective oxidation of alcohols to carbonyl compounds.
De Luca L, et al.
Organic Letters, 3(19), 3041-3043 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service