294934
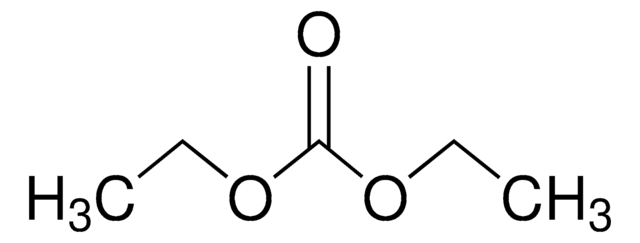
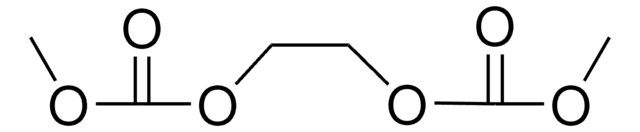
Dipropyl carbonate
99%
Synonym(s):
Di-n-propyl carbonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CH2CH2O)2CO
CAS Number:
Molecular Weight:
146.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
refractive index
n20/D 1.401 (lit.)
bp
167-168 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
SMILES string
CCCOC(=O)OCCC
InChI
1S/C7H14O3/c1-3-5-9-7(8)10-6-4-2/h3-6H2,1-2H3
InChI key
VUPKGFBOKBGHFZ-UHFFFAOYSA-N
Related Categories
General description
Thermal decomposition of dipropyl carbonate at 300-400°C yields carbon dioxide, alkene and alcohol. Dipropyl carbonate reacts with hydrous titanium dioxide (TiO2 ·nH2O, n=0.15-1.23) at 453-573K to yield titanium tetraalkoxides [Ti(OPrn)4]. N435-catalyzed transesterification of diethyl carbonate with dipropyl carbonate to form ethyl propyl carbonate has been reported.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
131.0 °F - closed cup
Flash Point(C)
55 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of titanium tetraalkoxides from hydrous titanium dioxideand dialkyl carbonates.
Suzuki E, et al.
Journal of Materials Chemistry, 7(10), 2049-2051 (1997)
Controlled lipase-catalyzed synthesis of poly (hexamethylene carbonate).
Jiang Z, et al.
Macromolecules, 40(22), 7934-7943 (2007)
The thermal decomposition of simple carbonate esters.
Cross JTD, et al.
Australian Journal of Chemistry, 29(7), 1477-1481 (1976)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service