All Photos(2)
About This Item
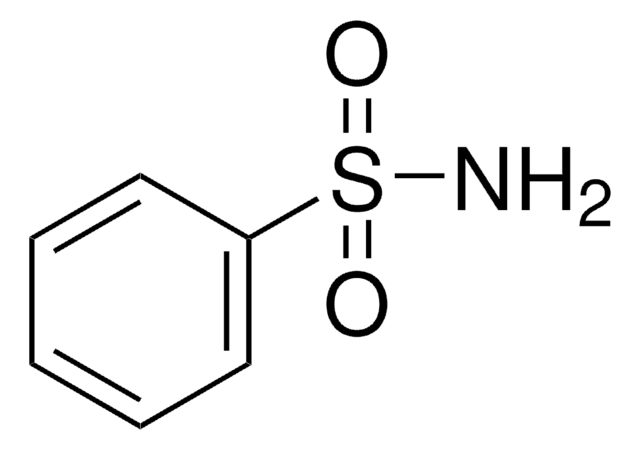
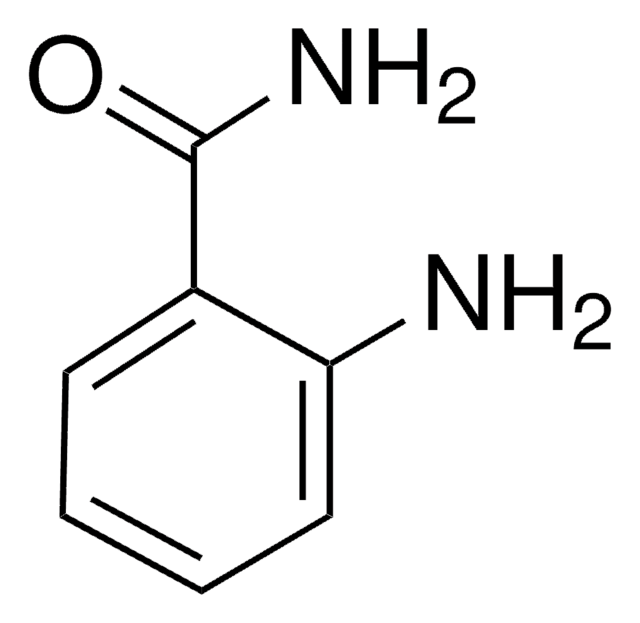
Linear Formula:
H2NC6H4SO2NH2
CAS Number:
Molecular Weight:
172.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
155-157 °C (lit.)
SMILES string
Nc1ccccc1S(N)(=O)=O
InChI
1S/C6H8N2O2S/c7-5-3-1-2-4-6(5)11(8,9)10/h1-4H,7H2,(H2,8,9,10)
InChI key
YAZSBRQTAHVVGE-UHFFFAOYSA-N
Gene Information
human ... CA1(759) , CA2(760) , CA5A(763) , CA5B(11238) , CA9(768)
mouse ... Car13(71934)
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
R Di Santo et al.
Farmaco (Societa chimica italiana : 1989), 52(6-7), 375-378 (1997-06-01)
The synthesis of pyrrolo[2,1-d][1,2,5]benzothiadiazepin-7(6H)-one 5,5-dioxide has been achieved by reaction between 2-(1H-pyrrol-1-yl)benzenesulfonamide and triphosgene. N-Ethylation of the tricyclic derivative afforded 6-ethylpyrrolo[2,1-d][1,2,5] benzothiadiazepin-7(6H)-one 5,5-dioxide, also obtained by the action of trifosgene on N-ethyl 2-(1H-pyrrol-1-yl)benzenesulfonamide. Preparation of pyrrole derivatives from 2-aminobenzenesulfonamide and
Robert Rönn et al.
Bioorganic & medicinal chemistry, 16(6), 2955-2967 (2008-01-16)
Inhibition of the hepatitis C virus (HCV) NS3 protease has emerged as an attractive approach to defeat the global hepatitis C epidemic. In this work, we present the synthesis and biochemical evaluation of HCV NS3 protease inhibitors comprising a non-natural
Nitrogen-15 nuclear magnetic resonance study of benzenesulfonamide and cyanate binding to carbonic anhydrase.
K Kanamori et al.
Biochemistry, 22(11), 2658-2664 (1983-05-24)
Laurence Josset et al.
PloS one, 5(10) (2010-10-20)
Classical antiviral therapies target viral proteins and are consequently subject to resistance. To counteract this limitation, alternative strategies have been developed that target cellular factors. We hypothesized that such an approach could also be useful to identify broad-spectrum antivirals. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service