227153
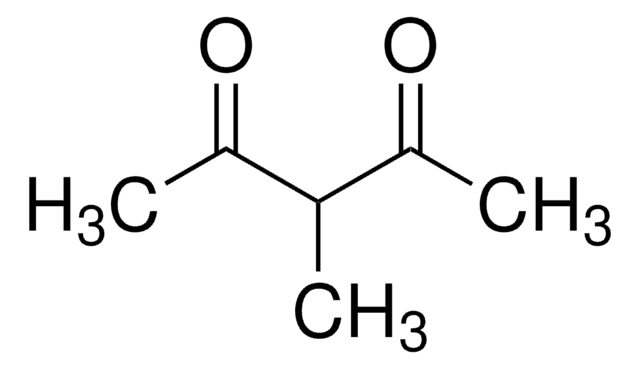
3-Chloro-2,4-pentanedione
97%
Synonym(s):
3-Chloroacetylacetone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3COCHClCOCH3
CAS Number:
Molecular Weight:
134.56
Beilstein:
605870
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.483 (lit.)
bp
49-52 °C/18 mmHg (lit.)
density
1.1921 g/mL at 20 °C (lit.)
functional group
chloro
storage temp.
2-8°C
SMILES string
CC(=O)C(Cl)C(C)=O
InChI
1S/C5H7ClO2/c1-3(7)5(6)4(2)8/h5H,1-2H3
InChI key
VLRGXXKFHVJQOL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The tautomeric properties of 3-chloro-2,4-pentanedione were studied using gas electron diffraction (GED) and quantum chemical calculations.
Application
3-Chloro-2,4-pentanedione was used in the synthesis of tetrathiafulvenyl-acetylacetonate (TTFSacacH), precursor of novel redox-active ligands.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
132.8 °F - closed cup
Flash Point(C)
56 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Natalya V Belova et al.
The journal of physical chemistry. A, 112(14), 3209-3214 (2008-03-07)
The tautomeric properties of alpha-chlorinated acetylacetone, 3-chloro-2,4-pentanedione CH3C(O)-CHCl-C(O)CH3, have been investigated by gas electron diffraction (GED) and quantum chemical calculations (B3LYP and MP2 approximations with different basis sets up to cc-pVTZ). Analysis of the GED intensities resulted in the presence
Julien Massue et al.
Inorganic chemistry, 44(24), 8740-8748 (2005-11-22)
The reaction of tris(alkylthio)tetrathiafulvalene thiolates with 3-chloro-2,4-pentanedione affords tetrathiafulvalene (TTF) moieties substituted by the acetylacetone function (TTFSacacH), precursors of novel redox-active ligands: the acetylacetonate ions (TTFSacac). These TTFSacacHs have been characterized by X-ray diffraction analyses, and similar trends have been
Almeqdad Y Habashneh et al.
Archiv der Pharmazie, 347(6), 415-422 (2014-03-13)
A new series of N1-(flavon-6-yl)amidrazones were synthesized by reacting the hydrazonoyl chloride derived from 6-aminoflavone with the appropriate sec-cyclic amines. The antitumor activities of these compounds were evaluated on breast cancer (MCF-7) and leukemic (K562) cell lines. Among the compounds
Jennifer A Jacobsen et al.
Journal of medicinal chemistry, 54(2), 591-602 (2010-12-30)
Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced hit rates ranging from 29% to 43% for five matrix metalloproteases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service