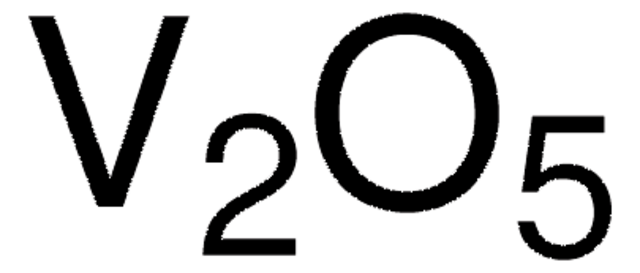213462
Sodium borohydride
ReagentPlus®, 99%
Synonym(s):
Sodium tetrahydridoborate
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NaBH4
CAS Number:
Molecular Weight:
37.83
EC Number:
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
form
powder
reaction suitability
reagent type: reductant
mp
>300 °C (dec.) (lit.)
SMILES string
[BH4-].[Na+]
InChI
1S/BH4.Na/h1H4;/q-1;+1
InChI key
YOQDYZUWIQVZSF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Sodium borohydride, also known as sodium tetrahydridoborate, is a highly basic boron hydride that is commonly used as a reducing agent. It reacts very slowly with water and acts as a catalyst and stabilizer.
Application
Sodium borohydride can be used as a reducing agent for the:
- Synthesis of cobalt (II, III) oxide (Co3O4) from cobalt chloride (CoCl2) via a co-reduction process.
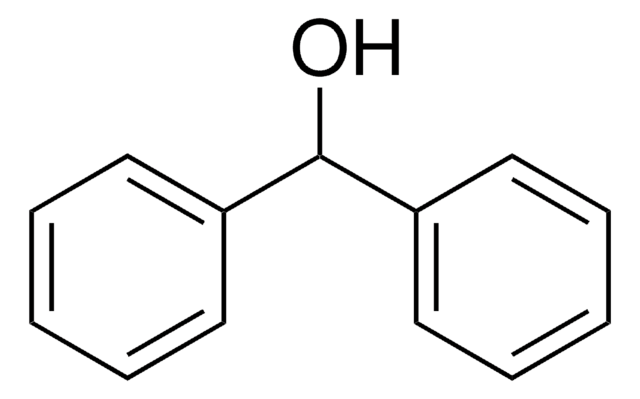
- Solvent-free reduction of aldehydes and ketones to alcohols in the presence of solid acid as an activator.
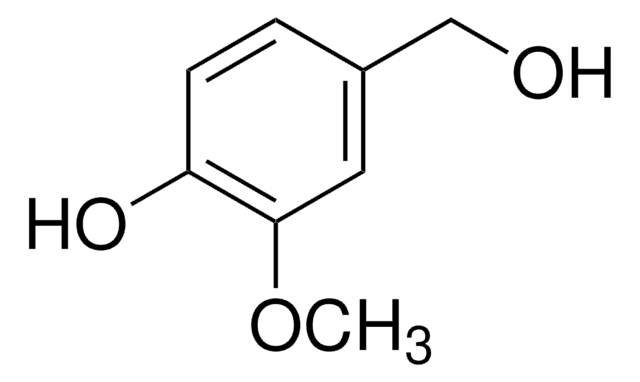
- Reductive amination of carbonyl compounds to secondary amines in the presence of silica-gel-supported sulfuric acid as a catalyst.
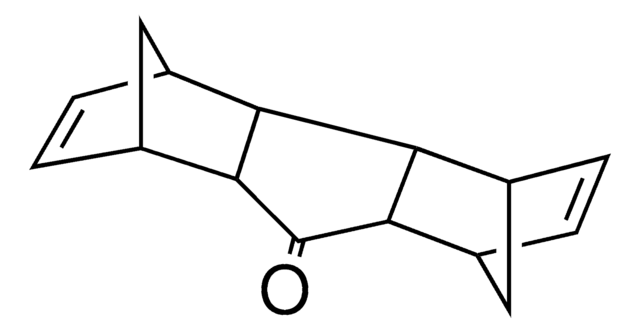
- Raney nickel-catalyzed reduction of aromatic nitro compounds to aromatic amines.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Repr. 1B - Skin Corr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dinesh Bhalothia et al.
Nanomaterials (Basel, Switzerland), 9(7) (2019-07-25)
Herein, ternary metallic nanocatalysts (NCs) consisting of Au clusters decorated with a Pt shell and a Ni oxide core underneath (called NPA) on carbon nanotube (CNT) support were synthesized by combining adsorption, precipitation, and chemical reduction methods. By a retrospective
Basavaiah, D. et al.
The Journal of Organic Chemistry, 64, 1197-1197 (1999)
Barbara Fogli et al.
Scientific reports, 9(1), 10095-10095 (2019-07-14)
While axons within the central nervous system (CNS) do not regenerate following injury, those in the peripheral nervous system (PNS) do, although not in a clinically satisfactory manner as only a small proportion of axons exhibit long-distance regeneration. Moreover, functional
See Wee Chee et al.
Nature communications, 10(1), 2831-2831 (2019-06-30)
At elevated temperatures, bimetallic nanomaterials change their morphologies because of the interdiffusion of atomic species, which also alters their properties. The Kirkendall effect (KE) is a well-known phenomenon associated with such interdiffusion. Here, we show how KE can manifest in
Lin, X.M. et al.
Chemistry of Materials, 11, 198-198 (1999)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service