All Photos(1)
About This Item
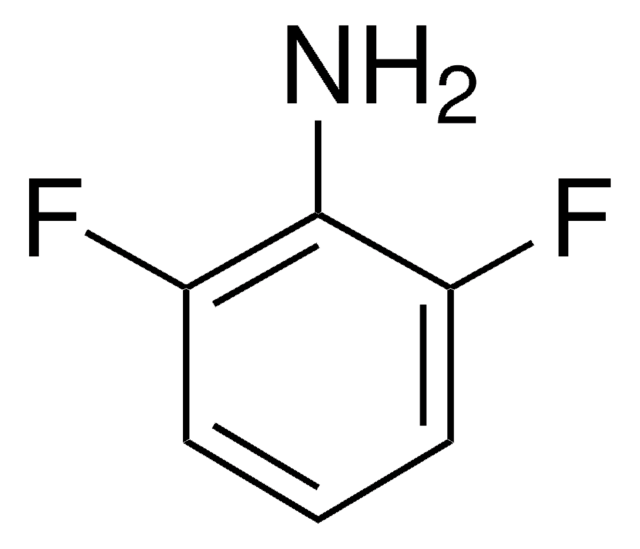
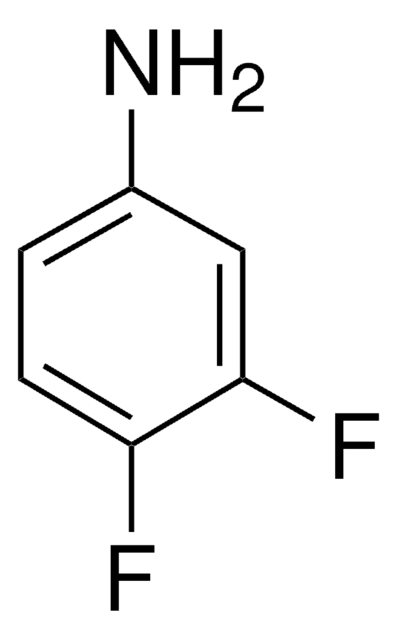
Linear Formula:
F2C6H3NH2
CAS Number:
Molecular Weight:
129.11
Beilstein:
2802549
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99.5%
refractive index
n20/D 1.513 (lit.)
bp
176-178 °C (lit.)
mp
11-13 °C (lit.)
density
1.288 g/mL at 25 °C (lit.)
functional group
fluoro
SMILES string
Nc1cc(F)ccc1F
InChI
1S/C6H5F2N/c7-4-1-2-5(8)6(9)3-4/h1-3H,9H2
InChI key
YNOOQIUSYGWMSS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,5-Difluoroaniline was used in the synthesis of 2,4-di-tert-butyl-6-[(2,5-difluorophenyl)iminomethyl]phenol. It was also used in ultrasound-assisted preparation of 1,4-diazabutadienes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
150.8 °F - closed cup
Flash Point(C)
66 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jing-Yu He et al.
Ultrasonics sonochemistry, 18(1), 466-469 (2010-08-28)
An ultrasound-assisted preparation of 1,4-diazabutadienes via smooth condensation of diketones with amines under solvent-free conditions is described. The generality of this method was examined by the synthesized N,N'-diaryl- and N,N'-dialkyl-1,4-diazabutadiene derivatives. In addition to experimental simplicity, the main advantages of
Omer Celik et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 11), o2786-o2786 (2009-01-01)
In the title Schiff base, C(21)H(25)F(2)NO, the dihedral angle between the aromatic rings is 27.90(5)° and an intramolecular O-H⋯N hydrogen bond occurs. In the crystal, the molecules are linked by C-H⋯O, C-H⋯N and C-H⋯F interactions.
I M Rietjens et al.
Chemico-biological interactions, 94(1), 49-72 (1995-01-01)
The in vivo metabolite patterns of 2,5-difluoroaminobenzene and of its nitrobenzene analogue, 2,5-difluoronitrobenzene, were determined using 19F NMR analysis of urine samples. Results obtained demonstrate significant differences between the biotransformation patterns of these two analogues. For the aminobenzene, cytochrome P450
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service