All Photos(3)
About This Item
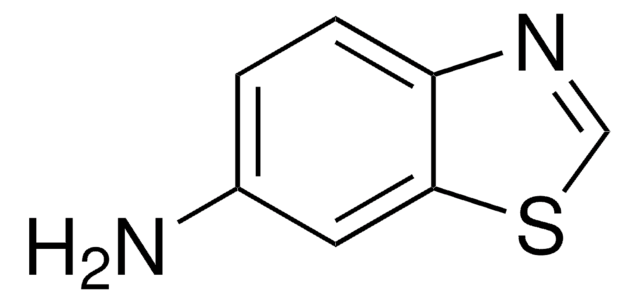
Empirical Formula (Hill Notation):
C7H5N3O2S
CAS Number:
Molecular Weight:
195.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
mp
247-249 °C (lit.)
functional group
nitro
SMILES string
Nc1nc2ccc(cc2s1)[N+]([O-])=O
InChI
1S/C7H5N3O2S/c8-7-9-5-2-1-4(10(11)12)3-6(5)13-7/h1-3H,(H2,8,9)
InChI key
GPNAVOJCQIEKQF-UHFFFAOYSA-N
General description
Voltammetric behavior of 2-amino-6-nitrobenzothiazole has been investigated using direct current voltammetry and differential pulse voltammetry.
Application
2-Amino-6-nitrobenzothiazole has been used:
- as model analyte for voltammetric determination of electrochemically reducible organic substances
- in the synthesis of 2-methyl-4-nitro-2H-pyrazole-3-carboxylic acid[2-(cyclohexanecarbonylamino)benzothiazol-6-yl]amide derivatives
- in the preparation of push-pull nonlinear optical chromophores containing thiazole and benzothiazole acceptors
- as a base in dye production by diazotation reaction
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Voltammetric determination of 2-amino-6-nitrobenzothiazole at two different silver amalgam electrodes.
Deylova D, et al.
Electrochimica Acta, 62, 335-340 (2012)
Masao Yoshida et al.
Bioorganic & medicinal chemistry letters, 15(14), 3328-3332 (2005-06-16)
Based on 2-methyl-4-nitro-2H-pyrazole-3-carboxylic acid[2-(cyclohexanecarbonylamino)benzothiazol-6-yl]amide (1), which shows selective cytotoxicity against tumorigenic cell lines, 2,6-dichloro-N-[2-(cyclopropanecarbonylamino)benzothiazol-6-yl]benzamide (13b) was designed and synthesized as a biologically stable derivative containing no nitro group. The highly potent derivative 13b exhibited excellent in vivo inhibitory effect on
Synthesis and characterization of thermally stable second-order nonlinear optical side-chain polyimides containing thiazole and benzothiazole push-pull chromophores.
Tambe SM, et al.
Opt. Mater., 31(6), 817-825 (2009)
Dana Deýlová et al.
Talanta, 102, 68-74 (2012-11-28)
New type of bismuth film electrode prepared by electrodeposition of bismuth film on a silver solid amalgam substrate (BiF-AgSAE) was tested as a sensor for voltammetric determination of electrochemically reducible organic substances using 2-amino-6-nitrobenzothiazole (ANBT) as a model analyte. Using
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service