187186
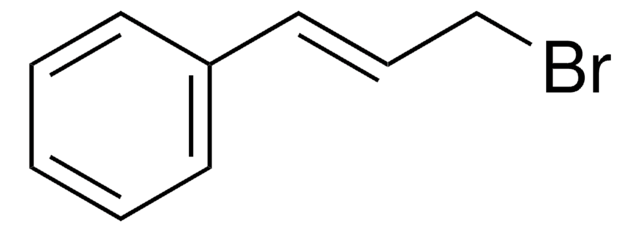
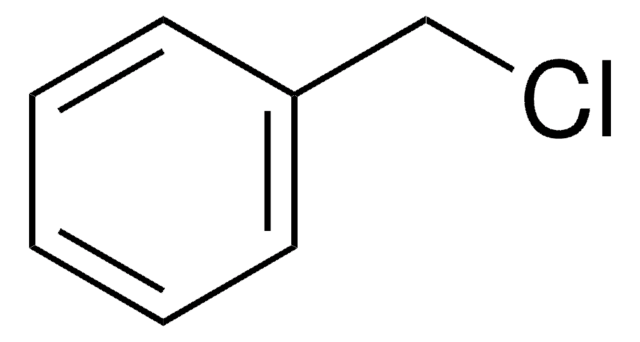
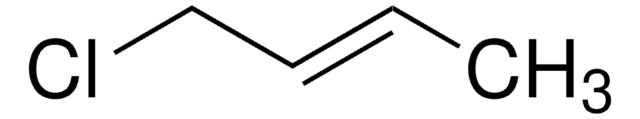
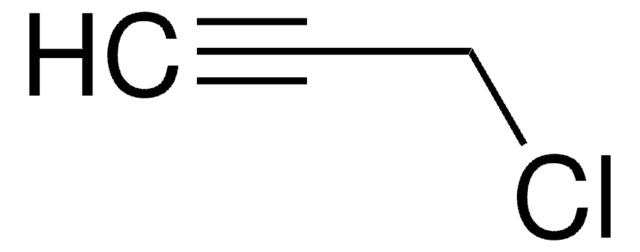
Cinnamyl chloride
95%
Synonym(s):
(3-Chloropropenyl)benzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=CHCH2Cl
CAS Number:
Molecular Weight:
152.62
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.584 (lit.)
bp
108 °C/12 mmHg (lit.)
mp
−19 °C (lit.)
density
1.096 g/mL at 25 °C (lit.)
functional group
chloro
phenyl
storage temp.
2-8°C
SMILES string
ClC\C=C\c1ccccc1
InChI
1S/C9H9Cl/c10-8-4-7-9-5-2-1-3-6-9/h1-7H,8H2/b7-4+
InChI key
IWTYTFSSTWXZFU-QPJJXVBHSA-N
Related Categories
General description
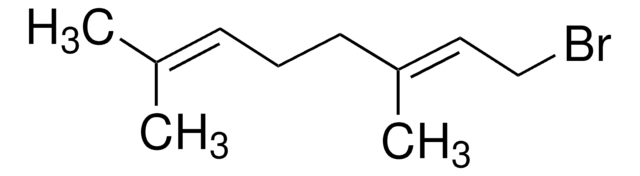
Cinnamyl chloride reacts regioselectively with aryl and alkenylgold(I) phosphanes in the presence of palladium catalyst in THF to afford the α-substitution product†.
Application
Cinnamyl chloride was used in the enantioselective total synthesis of helioporins C and E, bioactive marine diterpenes.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Muta. 2 - Repr. 2 - Skin Irrit. 2 - Skin Sens. 1B - STOT RE 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Wibke Lölsberg et al.
Organic letters, 14(23), 5996-5999 (2012-11-15)
A short and enantioselective total synthesis of helioporins C and E, which are bioactive marine diterpenes containing a serrulatane or amphilectane skeleton, was elaborated. The chirogenic step, i.e. a Cu(I)-catalyzed allylic alkylation of a cinnamyl chloride with methylmagnesium bromide, proceeded
Juan Carlos Rueda et al.
Polymers, 12(6) (2020-06-26)
Stiff thermosensitive hydrogels (HG) were synthesized by self-crosslinking free radical polymerization of N,N-dimethylacrylamide (DMAA) and N-isopropylacrylamide (NIPAAm), adjusting the degree of swelling by carboxylate-containing sodium acrylate (NaAc) or a 2-oxazoline macromonomer (MM). The formation of hydrogels was possible due to
Palladium-catalyzed cross-coupling reactions of organogold(I) phosphanes with allylic electrophiles.
Miguel Peña-López et al.
Organic & biomolecular chemistry, 10(8), 1686-1694 (2012-01-24)
Aryl and alkenylgold(I) phosphanes react regioselectively with allylic electrophiles such as cinnamyl and geranyl halides (bromide, chloride and acetates) under palladium catalysis in THF at 80 °C to afford the α-substitution product with moderate to high yields. When the reaction
Global Trade Item Number
| SKU | GTIN |
|---|---|
| S33295-1EA | |
| 187186-100G | 4061838758422 |
| 187186-10KG | |
| 187186-5G | 4061838758439 |
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service