All Photos(2)
About This Item
Empirical Formula (Hill Notation):
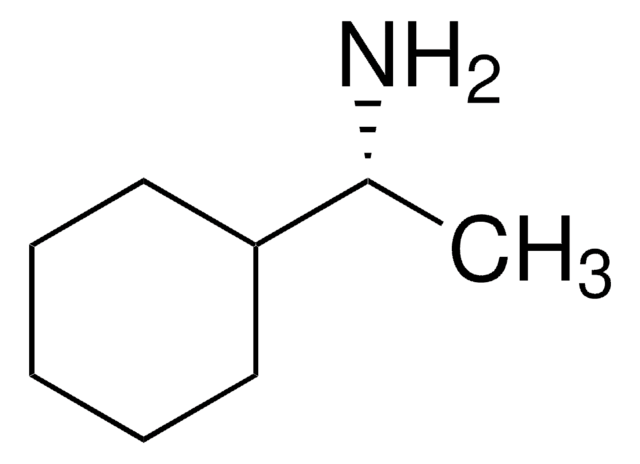
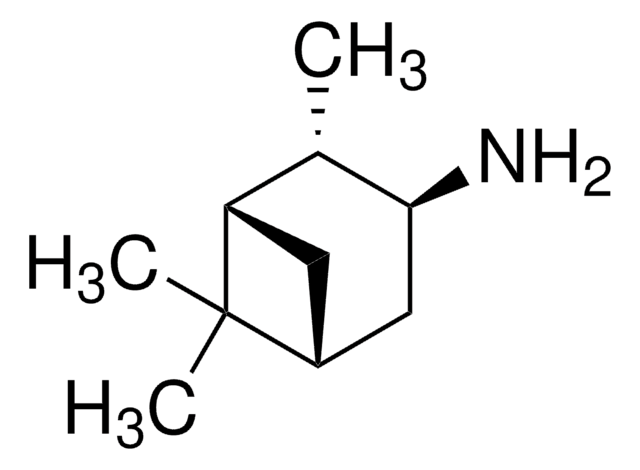
C10H19N
CAS Number:
Molecular Weight:
153.26
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
optical activity
[α]22/D −30.5°, neat
refractive index
n20/D 1.4877 (lit.)
bp
94-99 °C/27 mmHg (lit.)
density
0.915 g/mL at 25 °C (lit.)
functional group
amine
SMILES string
CC1(C)[C@@]2([H])CC[C@](CN)([H])[C@@]1(C2)[H]
InChI
1S/C10H19N/c1-10(2)8-4-3-7(6-11)9(10)5-8/h7-9H,3-6,11H2,1-2H3/t7-,8-,9-/m0/s1
InChI key
SYJBFPCQIJQYNV-CIUDSAMLSA-N
Related Categories
Application
(-)-cis-Myrtanylamine may be used:
- As a reactant to prepare N,N-bis((-)-cis-myrtanyl)butylene-2,3-diimine (BMDI), a bidentate diimine ligand that can form transition metal complexes for catalyzing asymmetric synthesis.
- As a building block to prepare imidazole derivatives, which are potent and selective cannabinoid receptor (CB2) antagonists.
- To prepare a chiral catalyst, which can catalyze asymmetric three-component Mannich reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Efficient catalysts for asymmetric Mannich reactions.
Rachwalski M, et al.
Organic & Biomolecular Chemistry, 11(25), 4207-4213 (2013)
Synthesis of new chiral diimine palladium (II) and nickel (II) complexes bearing oxazoline-and myrtanyl-based nitrogen ligands. Crystal structure of the C 2-symmetric complex [{(1R, 2S)-inda-box} PdCl 2].
Abu-Surrah AS, et al.
Polyhedron, 21(1), 27-31 (2002)
Synthesis and SAR of novel imidazoles as potent and selective cannabinoid CB 2 receptor antagonists with high binding efficiencies.
Lange JHM, et al.
Bioorganic & Medicinal Chemistry Letters, 20(3), 1084-1089 (2010)
Marco Busnelli et al.
British journal of pharmacology, 177(2), 328-345 (2019-10-18)
Fenretinide, a synthetic retinoid derivative first investigated for cancer prevention and treatment, has been shown to ameliorate glucose tolerance, improve plasma lipid profile and reduce body fat mass. These effects, together with its ability to inhibit ceramide synthesis, suggest that
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service