158755
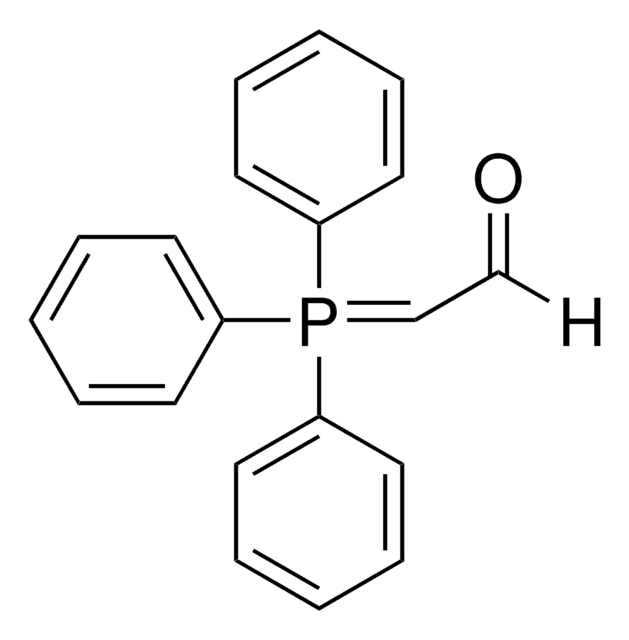
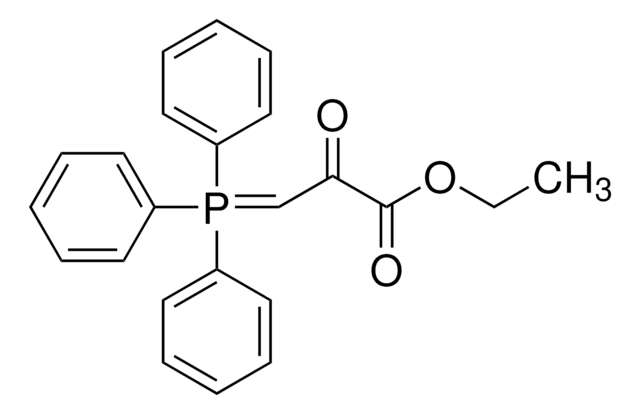
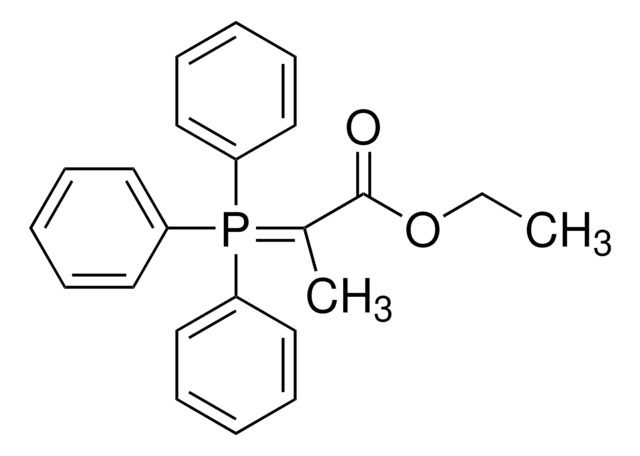
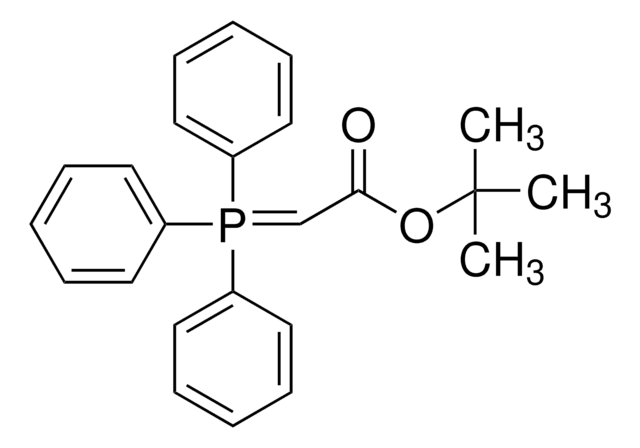
1-(Triphenylphosphoranylidene)-2-propanone
99%
Synonym(s):
(2-Oxopropylidene)triphenylphosphorane, Acetylmethylene-triphenylphosphorane, Methyl (triphenylphosphoranylidene)methyl ketone, NSC 407394
About This Item
Recommended Products
Quality Level
Assay
99%
form
solid
reaction suitability
reaction type: C-C Bond Formation
mp
203-205 °C (lit.)
functional group
ketone
phosphine
SMILES string
CC(=O)C=P(c1ccccc1)(c2ccccc2)c3ccccc3
InChI
1S/C21H19OP/c1-18(22)17-23(19-11-5-2-6-12-19,20-13-7-3-8-14-20)21-15-9-4-10-16-21/h2-17H,1H3
InChI key
KAANTNXREIRLCT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Cascade reactions of enals for enantioselective synthesis of indane derivatives
- Enantioselective conjugate addition for synthesis of α-branched indoles
- Synthesis of 1,2-dioxanes with antitrypanosomal activity
- Asymmetric allylboration for enantioselective synthesis of (+)-awajanomycin
- Domino Suzuki / Heck coupling reactions for preparation of fluorenylidenes
- Synthesis of amphibian pyrrolizidine alkaloids via allylic aminations
- Synthesis of silicon-containing acyclic dienone musk odorants
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service