15151
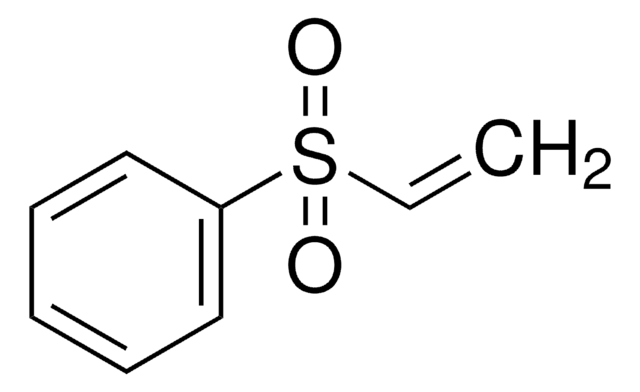
1,1-Bis(phenylsulfonyl)ethylene
≥98.0% (CH)
Synonym(s):
(Ethene-1,1-diyldisulfonyl)dibenzene, 1,1-Bis(benzenesulfonyl)ethylene, 1,1-Bis(phenylsulfonyl)ethene, 1,1′-[Ethenylidenebis(sulfonyl)]bis[benzene]
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12O4S2
CAS Number:
Molecular Weight:
308.37
Beilstein:
2056786
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (CH)
form
powder
mp
124-126 °C
functional group
sulfone
SMILES string
C=C(S(=O)(=O)c1ccccc1)S(=O)(=O)c2ccccc2
InChI
1S/C14H12O4S2/c1-12(19(15,16)13-8-4-2-5-9-13)20(17,18)14-10-6-3-7-11-14/h2-11H,1H2
InChI key
KABQEPJVQFXVIN-UHFFFAOYSA-N
Application
1,1-Bis(phenylsulfonyl)ethylene was used in the preparation of α,α-disubstituted alpha-amino acid derivatives.
Other Notes
A synthetic equivalent of the ethylene 1,2-dipole; Dienophile for Diels-Alder reactions, review; A useful synthon for neutral homologation of ketones.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
O. De Lucchi et al.
Tetrahedron Letters, 25, 3647-3647 (1984)
O. De Lucchi et al.
Tetrahedron Letters, 25, 3643-3643 (1984)
Andrea-Nekane R Alba et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 16(18), 5354-5361 (2010-03-09)
A new, easy, and highly enantioselective method for the synthesis of quaternary alpha-alkyl-alpha-amino acids based on organocatalysis is reported. The addition of oxazolones to 1,1-bis(phenylsulfonyl)ethylene is efficiently catalyzed by simple chiral bases or thioureas. The reaction affords alpha,alpha-disubstituted alpha-amino acid
Xianghong Liu et al.
Scientific reports, 4, 7452-7452 (2014-12-17)
With Fe2O3 as a proof-of-concept, free-standing nanomembrane structure is demonstrated to be highly advantageous to improve the performance of Li-ion batteries. The Fe2O3 nanomembrane electrodes exhibit ultra-long cycling life at high current rates with satisfactory capacity (808 mAh g(-1) after 1000
O. De Lucchi et al.
Tetrahedron, 44, 6755-6755 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service