101346
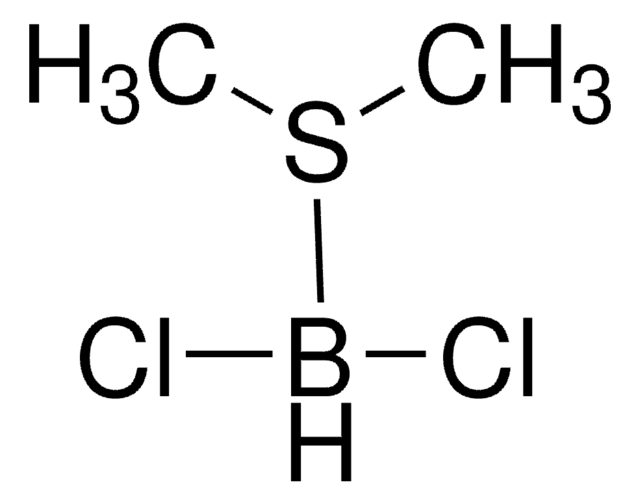
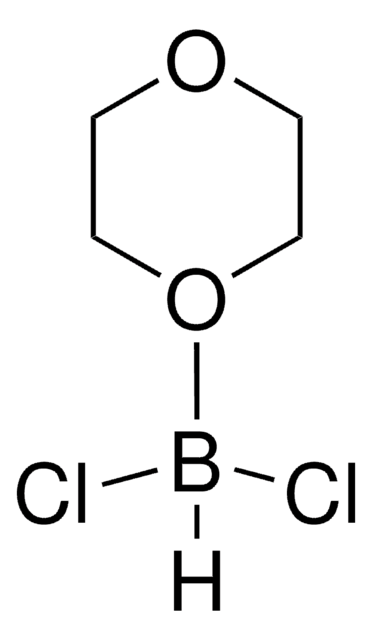
Dichlorophenylborane
97%
Synonym(s):
NSC 93889, Phenylboron dichloride, Phenyldichloroborane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H5BCl2
CAS Number:
Molecular Weight:
158.82
Beilstein:
2243576
MDL number:
UNSPSC Code:
12352002
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reagent type: reductant
refractive index
n20/D 1.545 (lit.)
bp
66 °C/11 mmHg (lit.)
density
1.224 g/mL at 25 °C (lit.)
SMILES string
ClB(Cl)c1ccccc1
InChI
1S/C6H5BCl2/c8-7(9)6-4-2-1-3-5-6/h1-5H
InChI key
NCQDQONETMHUMY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Catalyst for:
- Antiproliferative macrolide and cell migration inhibitor lactimidomycin
- Enantioselective synthesis of polyketide segments using vinylogous Mukaiyama aldol reactions
- Enantioselective Diels-Alder cycloadditions after its reaction with allo-threonine derivatives
- Stereoselective alkylative ring opening of cyclic anhydrides
- Cross-metathesis reaction of amino derivatives with olefins
- Asymmetric acetate aldol reactions
- The reaction of aryl aldehydes with styrene and (E)-β-methylstyrene
Dichlorophenylborane can be used as a catalyst for:
- The synthesis of an antiproliferative macrolide and cell migration inhibitor lactimidomycin.
- Enantioselective synthesis of polyketide segments using vinylogous Mukaiyama aldol reactions.
- Enantioselective Diels-Alder cycloadditions after its reaction with allo-threonine derivatives.
- Stereoselective alkylative ring opening of cyclic anhydrides.
- Cross-metathesis reaction of amino derivatives with olefins.
- Asymmetric acetate aldol reactions.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Borheterocyclen, 2. Mitt.
Pailer M and Huemer H
Monatshefte fur Chemie / Chemical Monthly, 95(2), 373-378 (1964)
Total syntheses and biological reassessment of lactimidomycin, isomigrastatin and congener glutarimide antibiotics.
Micoine K, et al.
Chemistry?A European Journal , 19(23), 7370-7383 (2013)
Synthesis of a New N-Acetyl Thiazolidinethione Reagent and Its Application to a Highly Selective Asymmetric Acetate Aldol Reaction.
Zhang Y and Sammakia T
Organic Letters, 6(18), 3139-3141 (2004)
Oxazaborolidinone-Promoted Vinylogous Mukaiyama Aldol Reactions.
Simsek S, et al.
Organic Letters, 9(26), 5637-5639 (2007)
Synthesis of 1-Indanonyl Oxepanes.
Chang M Y and Lee N C
Synlett, 23(06), 867-872 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service