T2036
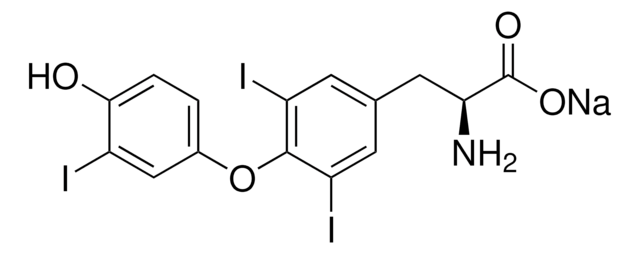
apo-Transferrin human
powder, BioReagent, suitable for cell culture, ≥98% (agarose gel electrophoresis)
Synonym(s):
Human transferrin, Siderophilin
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
product line
BioReagent
Quality Level
Assay
≥98% (agarose gel electrophoresis)
form
powder
mol wt
76-81 kDa
concentration
~25 mM
technique(s)
cell culture | mammalian: suitable
impurities
HIV and HBsAg, source material tested negative
endotoxin, tested
solubility
H2O: 50 mg/mL
cation traces
Fe: ≤0.005%
UniProt accession no.
shipped in
ambient
storage temp.
−20°C
Gene Information
human ... TF(7018)
Looking for similar products? Visit Product Comparison Guide
General description
Transferrin, majorly synthesized in the liver, is an abundant glycoprotein in the serum. The members of the transferrin superfamily have similar polypeptide folding patterns. Transferrin contains N- and C-terminal iron-binding homologous domains. Each of these domains is split into two subdomains having binding sites for iron and anions within the inter-subdomain cleft. The binding cleft opens and closes with iron releasing and iron binding. apo-Transferrin is an iron-free protein that arises after transferrin dissociates from its receptor. This product can be supplemented with iron or used to bind free iron present in media.
Application
apo-Transferrin human has been used:
- to culture human primary pancreatic endothelial cells, Het1As (non-tumorous esophagus cells), and immortalized human colonic epithelial cells (HCEC-1CT)
- to culture α mouse liver 12 (AML-12) (mature hepatocytes) cells
- as a source for human apo-transferrin for purification before crystallography
Biochem/physiol Actions
Transferrin is responsible for transporting iron from the sites of absorption and storage to the tissue cells. It maintains the levels of iron in biological fluids. The levels of transferrin may indicate the total iron-binding capacity (TIBC). Transferrin supplies the required iron for incorporation into hemoglobin within RBCs in the bone marrow. Iron deficiency causes an increase in the levels of transferrin. Pregnancy and estrogen administration can also raise the levels of transferrin. Chronic liver disease, renal insufficiency, malnutrition, and protein-losing enteropathies reduce the synthesis of transferrin.
Other Notes
Non-heme iron-transport protein.
Disclaimer
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Justin P Curtin et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 23(3), 471-480 (2018-04-07)
The presence of ionic titanium in the serum of patients with titanium implants is currently unexplained. This is presumed due to corrosion, and yet the serum titanium concentration measured in patients is far greater than that predicted by its solubility.
Hanseul Park et al.
Nature communications, 14(1), 802-802 (2023-02-14)
Alzheimer's disease (AD) is associated with progressive neuronal degeneration as amyloid-beta (Aβ) and tau proteins accumulate in the brain. Glial cells were recently reported to play an important role in the development of AD. However, little is known about the
Kasumi Murai et al.
Nature communications, 13(1), 6206-6206 (2022-10-21)
Aging normal human oesophagus accumulates TP53 mutant clones. These are the origin of most oesophageal squamous carcinomas, in which biallelic TP53 disruption is almost universal. However, how p53 mutant clones expand and contribute to cancer development is unclear. Here we
Orsolya Dömötör et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 27(3), 315-328 (2022-03-05)
Solution speciation and serum protein binding of selected In(III) complexes bearing O,O and O,N donor sets were studied to provide comparative data for In(III) and analogous Ga(III) complexes. Aqueous stability of the In(III) complexes of maltol, deferiprone, 8-hydroxyquinoline (HQ) and
Daniel Sommer et al.
Frontiers in molecular neuroscience, 15, 894230-894230 (2022-07-02)
Amyotrophic Lateral Sclerosis (ALS) is an incurable neurodegenerative disease characterized by dysfunction and loss of upper and lower motor neurons (MN). Despite several studies identifying drastic alterations affecting synaptic composition and functionality in different experimental models, the specific contribution of
Related Content
Generic QA Testing Page
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service