P3644
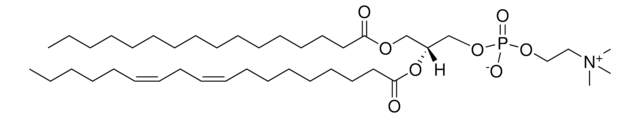
L-α-Phosphatidylcholine
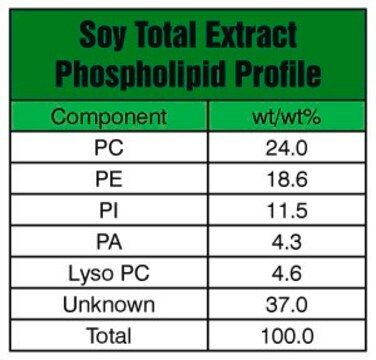
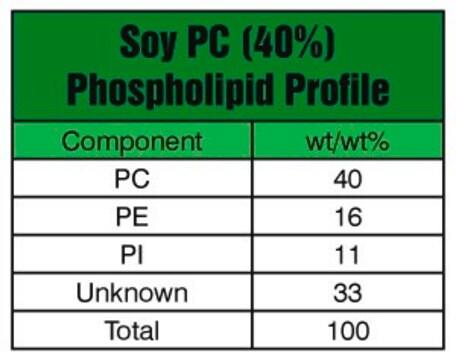
from soybean, Type IV-S, ≥30% (enzymatic)
Synonym(s):
1,2-Diacyl-sn-glycero-3-phosphocholine, 3-sn-Phosphatidylcholine, L-α-Lecithin, Azolectin, PC
About This Item
Recommended Products
biological source
soybean
Quality Level
type
Type IV-S
form
solid
concentration
≥30% (enzymatic)
solubility
chloroform: soluble 100 mg/mL, clear to slightly hazy, yellow to orange
functional group
phospholipid
lipid type
phosphoglycerides
shipped in
ambient
storage temp.
−20°C
SMILES string
[P](=O)([O-])(OC[C@H](OC(=O)CCCCCCC\C=C/C\C=C/CCCCC)COC(=O)CCCCCCCCCCCCCCC)OCC[N+](C)(C)C
InChI
1S/C42H80NO8P/c1-6-8-10-12-14-16-18-20-21-23-25-27-29-31-33-35-42(45)51-40(39-50-52(46,47)49-37-36-43(3,4)5)38-48-41(44)34-32-30-28-26-24-22-19-17-15-13-11-9-7-2/h14,16,20-21,40H,6-13,15,17-19,22-39H2,1-5H3/b16-14-,21-20-/t40-/m1/s1
InChI key
JLPULHDHAOZNQI-ZTIMHPMXSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a component of human erythroid massive amplification culture (HEMAdef) medium for clinical expansion of human erythroblasts
- in the chloride efflux assay to examine the chloride permeability of lipid vesicles conferred by soluble CLIC1
- in an assay to measure solubilized wax synthase activity
- as a component of buffer A used for the resuspension of the cholate-solubilized catalytic unit of adenylate cyclase
- as a cryoprotectant for freezing ruminant sperm
- as a substrate for the estimation of phospholipase activity
- to make liposomes to assess their efficiency of recruitment of ARF (ADP ribosylation factor) and AP-1 (adaptor protein)
- in the preparation of the isolated catalytic unit of soluble adenylate cyclase
Biochem/physiol Actions
Preparation Note
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service